Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Aurones are part of the wide family of polyphenols. More specifically, they may be acknowledged as the lower structural counterparts of the best-known flavones, a subclass of flavonoids.
- aurones
- polyphenols
- flavonoids
1. Aurones as Natural Dyes
The color of flowers is the keystone of the evolutionary process that marked the transition from a random pollination, mediated exclusively by the wind, to the use of a vector, that is, insects, birds, and lizards. Being attracted from the nectar of flowers, vectors confer an advantage to the plant world, which may benefit from a greater chance of survival thanks to the passage of pollen from one plant to another and from one place to another. This is why, over the course of time, flowers and plants in general have equipped themselves to attract more and more vectors, in particular by expressing pigments that match the perceptions of these animals [11][1].
When walking in nature, the characteristic that most catches the eye is a bright yellow color. This is due to aurones and turns out to be more attractive than other shades of yellow given by other parent compounds such as flavonols. Actually, overall, aurones can be acknowledged as the brightest polyphenol pigments in the yellow color range, like anthocyanins are for the red/purple spectrum. The visual contrast that is perceived is the result of different UV absorption spectra. Flavones, isoflavones, and flavanones exhibit UV absorption at 350 nm and, for this reason, they are not characterized by any visible color. Instead, aurones have an absorption spectrum in the 390–430 nm range, thus resulting in a more intense colors than the parent chalcones, showing UV absorption in the 365–390 nm range, and flavonols, having 350–390 nm as the reference range [12][2].
Being characterized by a brilliant color, aurones play a key role in pollination, attracting vectors towards the flowers and to the pollen. Their distribution in the petals of flowers is generally unique. For example, in the case of A. majus, the production of aurones is limited to the upper side of the petal, but also affects two additional stripes at the level of the throat, mainly surrounded by a magenta or pink color due to the presence of anthocyanins. The brilliant stripes have the crucial role of warning the insect on the place of nectar [13][3]. There is also the case where the flowers appear completely yellow, but they this is not so: the contrast is perceived as two-tone by UV-sensitive insects, and these different colors act as a guide for nectar, as was observed in Bidens ferulifolia and Coreopsis gigantea. This is an example of the evolutionary adaptation of flowers, to better attract pollinating insects such as bees [14][4].
However, as ever, there is always the exception that proves the rule: a particular aurone does exist, 3′,5′-dihydroxy-4′-methoxyaurone (9, Figure 1), inducing a red/scarlet color in the nectar of the flowers of specific plants such as the Mauritian Nesocodon mauritianus. This is an almost unique exception as this compound provides a color that is not bright yellow. However, the special shade is thought to be due to the particularly alkaline pH of the nectar, which causes the deprotonation of the hydroxyl group of the aurone, leading to a different electronic delocalization [15][5].
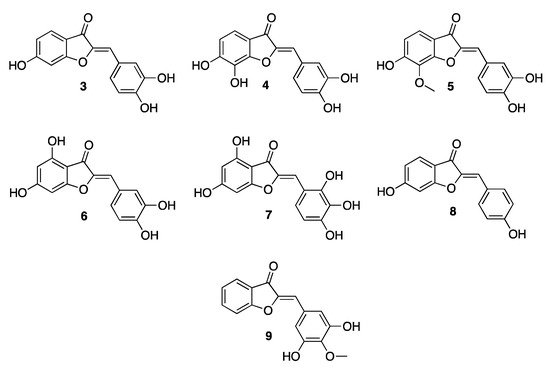
Figure 1. Chemical structures of 4-deoxy- and 4-hydroxy-aurones.
From a commercial point of view, the color of the flowers is of great interest. In this regard, the use of aurones has been thought of to create transgenic plants endowed with bright yellow flowers through genetic engineering approaches. By investigating the natural biosynthetic pathways of Torenia hybrida, belonging to the Scrophulariaceae family, Tanaka and co-workers demonstrated that the A. majus aureusidin synthase (AmAS1) is the key enzyme catalyzing aurone biosynthesis from chalcones. However, to accomplish aurone biosynthesis and produce yellow-colored flowers in vivo, AmAS1 must work in tandem with chalcone 4′-O-glucosyltransferase (4′CGT). Actually, the co-expression of both the AmAS1 and the 4′CGT genes turned out to be sufficient for the accumulation of aureusidin-6-O-glucoside in the flowers of transgenic plants, thus opening up the obtainment of novel bright-yellow flowers for plant species lacking this color variant [16][6].
2. Aurones as Functional Agents
By virtue of their poly-hydroxylated nature, aurones are able to quench reactive oxygen species (ROS), thus showing antioxidant properties. In analogy with the higher homologous flavones, the main mechanism by which aurones exert their protective role is by transferring an H atom to ROS, thus becoming radical species [17][7]. In addition, they may also transfer a single electron, giving rise to a radical cation. The H atoms of the hydroxy groups in both positions 3′ and 4′ of the benzylidene residue are considered to be the first shield to the attack of ROS. Once formed, the phenoxy radical may be easily stabilized thanks to the assistance of the carbon atoms in positions 2 and 3 of the nucleus, and also by the exocyclic oxygen atom in position 3, thus allowing the radical to be less prone to act as a pro-oxidant [18][8]. Accordingly, a high potential for ROS scavenger activity has been acknowledged for all the aurones showing a 4′-hydroxy-, as well as a 3′,4′-dihydroxy- and also a 3′,4′,5′-trihydroxy- substitution patterns. Thanks to a quantum chemical investigation, Senthil Kumar and co-workers demonstrated the high antioxidant properties of bracteatin (7, Figure 1), whose poly-substitution pattern on the pendant benzylidene residue allows the lowest energy for both the H-atom and electron transfer mechanism [19][9].
Aurones have proved to be effective against Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae, Proteus vulgaris, and Mycobacterium tuberculosis, thus showing a broad-spectrum antibacterial activity. In addition to natural derivatives, a number of synthetic aurones were also developed as active agents, highlighting the importance of a diversified 2-substitution pattern to achieve a relevant efficacy. In particular, enlargement of the aromatic area in this position, as in derivatives 10 and 11 (Figure 2), provided the most performing compounds, showing minimal inhibitory concentrations (MICs) in the micromolar/submicromolar range against several pathogen bacteria. Similar functional results were also achieved when the benzofuranone core was replaced with the bioisosteric indolone, as in derivative 12 (Figure 2) [20,21,22][10][11][12].
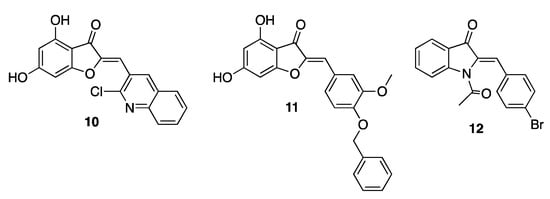
Figure 2. Representative synthetic antibacterial aurones.
As for the antifungal properties, aurones are mainly effective against Aspergillus fumigatus, Aspergillus niger, Trichoderma virdie, and Penicillium chrysogenum. Investigations into Candida albicans have demonstrated once more the importance of the 2-arylidene moiety for the antifungal activity. Derivatives 13 and 14 counteracted Candida spp., also displaying anti-biofilm activity for mid-maturation growth [23][13]. Aurones demonstrated insecticidal activity when tested against the larvae of Spodoptera litura [24][14]. In this case, the best activity was obtained in the presence of methoxylated derivatives. In particular, the 3′,4,4′,6 tetramethoxy-aurone 15 (Figure 3) is the compound that displayed the strongest insecticidal activity among the tested ones, thus highlighting positions 4, 6, 3′, and 6′ of the nucleus as those worth substituting with a view to implementing the activity. On the contrary, when the methoxy substituents were inserted in position 5 or 5′ the activity proved to decrease. Interestingly, the 3′,4,4′,6 tetramethoxy-aurone is also one of the main constituents isolated from the extract of Cyperus radians, an example of the Cyperaceae family that produce poly-methylated aurones as part of the body’s chemical defense system.
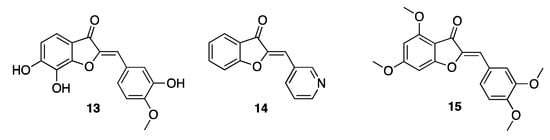
Figure 3. Representative natural and synthetic antifungal aurones.
The anti-malarian activity of a number of aurone derivatives has been demonstrated by several authors, who investigated both natural derivatives and synthetic analogues [25,26,27][15][16][17]. Analyses of structure–activity relationships have highlighted the importance of the substitution patterns in positions 4 and 6 of the benzofurane ring. In particular, the best in vitro anti-malarian efficacy was obtained in the presence of a halogen atom in position 4 of the nucleus and/or an amino group in position 6. Significantly, all the compounds turned out to be non-cytotoxic when tested in human cells. However, when the best performing derivative was tested in vivo in laboratory animals stricken by Plasmodium falciparum, the results were rather disappointing. Actually, as often occurs in the case of natural poly-hydroxylated derivatives, the functional efficacy observed in in vitro tests is hardly replicated in in vivo models, due to bioavailability limits that characterize this kind of compound.
Many aurones have also been evaluated for their inhibitory activity against the glycoprotein neuraminidase, which is involved in the infection processes of the most common influenza viruses. In this case, the analysis of the structure–activity relationship showed that the key structural elements of the natural compounds, represented by a hydroxyl group in position 4 or 6 of the nucleus and a double bond in position 2, are essential for the activity [28][18].
In 2011, some aurones were identified as inhibitors of the RNA polymerase RdRp, exploited by hepatitis C virus (HCV) for replication. Due to the extreme heterogeneity of the viral genome of the causative agent of hepatitis C, to date it has not yet been possible to develop a vaccine. Indeed, eight genotypes of HCV are currently known, each differing by 30% in nucleotide sequence [29][19]. For many years, the weak points of HCV have been found to make the infection, if not inert, at least less dangerous. In this regard, the research community has recently been focusing on RdRp, which is a key enzyme for viral replication [30][20]. Nucleoside and nucleotide analogues have been shown to target the active site of RdRp, but also non-nucleoside derivatives have been disclosed as allosteric inhibitors. Regarding aurones, aureusidin 6 proved to inhibit RdRp potently, showing an IC50 value of 5.2 μM. Thanks to the use of a classical medicinal chemistry approach, it has been possible to identify both substitutes and ring positions conferring to aurones the best inhibitory potency. The presence of hydroxy groups in positions 4 and 6 of the nucleus turned out to be crucial for the activity. On the contrary, replacement of the hydroxy groups with methoxy substituents in the same positions resulted in a loss of activity. Different aromatic substituents were also investigated in position 2 of the benzofuran ring, demonstrating that the insertion of an indole nucleus in this position, as in compound 16 (IC50 2.2 μM, Figure 4), significantly ameliorated the antiviral activity of the natural 6 [31][21]. Remarkable efficacy was also seen in the case of pseudodimeric compounds such as derivative 17 (IC50 1.3 μM, Figure 4) [32][22]. Aurones displaying the most promising RdRp inhibitory activity did not show any cytotoxic effect on human cells, thus showing that they are prominent candidates for the obtainment of anti-HCV agents.
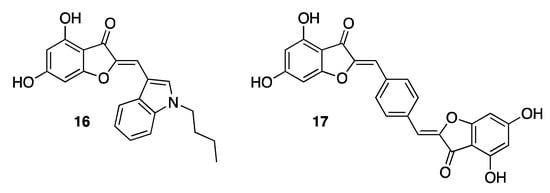
Figure 4. Representative examples of synthetic anti-HCV aurones.
Aurones and their derivatives have also been described as effective anti-inflammatory agents [6][23]. Actually, they are reported to inhibit the production of key cytokines such as TNF-α (tumor necrosis factor-alpha) and IL-6 (interleukin-6), released in most inflammatory processes and involved in many diseases such as autoimmune ones, diabetes, atherosclerosis, and cancer. Sulfuretin counteracts the activities of nitric oxide (NO) and prostaglandin E2 (PGE2), both pro-inflammatory molecules [32][22]. The presence of a hydroxy group in position 6 of the nucleus boosts the inhibition of PGE2 production, while its replacement with a methoxy group potentiates the inhibition of NO production. In any case, both substituents are crucial to combine the highest anti-inflammatory activity with the lowest toxicity. A number of sulfuretin derivatives have also been described, produced by modifying either the benzo-fused ring or the 2-benzylidene residue, or even both, which proved to be better in fighting inflammation. For example, the insertion of the 6-hydroxy substituent into a dihydropyran residue, as in compound 18 (Figure 5) [33][24], or the replacement of the 2-benzylidene residue with an heteroaryl moiety, as in compound 19 (Figure 5) [34][25].
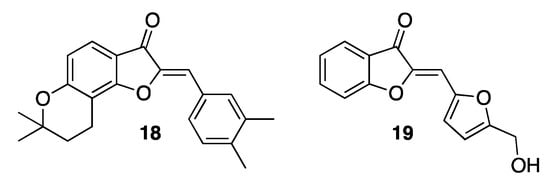
Figure 5. Representative examples of synthetic anti-inflammatory aurones.
However, most of the functional studies described by the literature aim at demonstrating the anti-cancer potential of aurones. Chemotherapy has always been the most powerful weapon at our disposal in the fight against cancer. Nevertheless, due to its high toxicity and the severe implications of resistance to the known anti-cancer drugs, the development of magic bullets for molecular targeted therapy has been progressing more and more. This is where aurones and their potential as anti-cancer agents come into play. The versatility of these natural compounds lies in the simplicity of their structure, which enables them to interact with key enzymes involved in tumor development. The first ever recorded antitumor activity of aurones has been described by Huang and co-workers, who demonstrated the ability of hamiltrone 20 (Figure 6), the 4,5,6-trimethoxy-substituted aurone obtained from the shrub Uvaria hamiltonii, to modulate the scissoring activity against the double-stranded DNA, thus damaging the DNA of proliferating cells [35][26]. From then on, a number of scientific reports have been published, thoroughly reviewed by Alsayari and co-workers, testifying to the ability of both aurones and their regioisomes isoaurones to interact with several key cancer targets.
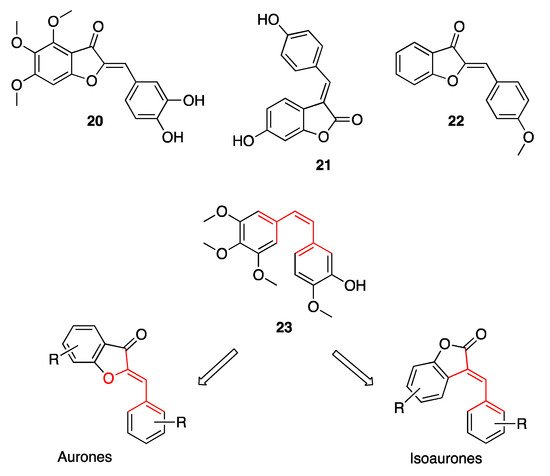
Figure 6. Representative examples of aurones endowed with anti-tumor efficacy.
Suzuki and co-workers demonstrated the anti-tumor efficacy of isoaurostatin 21 (Figure 6), the fungal metabolite of Thermomonospora alba, as the result of its inhibitory activity against Topoisomerase I [36][27], while Priyadarshani and co-workers reported on the ability of (Z)-2-(4-methoxybenzylidene)benzofuran-3(2H)-one 22 (Figure 6) to inhibit Topoisomerase II [37][28]. Overall, the benzofuran-3(2H)-one scaffold gave evidence these key enzymes regulating DNA replication and transcription, once decorated with appropriate substituents on the pendant phenyl ring. In any case, the reference pharmacophoric structure of both aurones and isoaurones, characterized by two main aromatic areas connected by a double bond having Z geometry, has been deemed by several authors to overlap the one of combrestatin A-4 23 (Figure 6), a well-known tubulin polymerization inhibitor. This would explain the anti-cancer activity shown by several natural and synthetic aurones, which proved to interact at the colchicine binding site of tubulin, thus arresting cell cycle at the G2/M level.
Both aurones and isoaurones, as well as the synthetic benzofuranone derivatives closely related to them, have also been reported to interact with additional anti-tumor targets. These include the serine/threonine cyclin-dependent kinases CDK 1 and 2, playing a key role in cell cycle regulation [38][29], the Hypoxia-inducible Factor-1 (HIF-1), a marker reflecting angiogenesic activity in cancer cells [39[30][31],40], but also protein kinases such as fibroblast growth factor receptor, FGFR, which is involved in cancer development and progression [41][32], and sphingosine kinase, whose overexpression has been associated with tumor angiogenesis and resistance to radiation and chemotherapy [42][33]. Examples of synthetic aurones have also been described for their ability to dissipate the hyperpolarization of mitochondrial membranes of cancer cells, arresting cell cycle [43][34]. Instead, contrasting results were obtained against histone deacetylases (HDACs), a family of enzymes that, once mutated or overexpressed, induce the aberrant expression of genes involved in cell proliferation and apoptosis. Indeed, while Zwick and co-workers claimed an HDAC inhibitory activity in the micromolar range for a number of poly-hydroxylated aurones [44][35], Itoh and co-workers warned against the activity displayed by those aurones bearing a catechol fragment on the 2-benzylidene residue [45][36]. Actually, according to the authors, this structural motif proved to interfere with the proper functioning of the in vitro assay components, thus causing false positives.
The development of the ideal anti-tumor agent cannot ignore the multi-drug resistance that cancer cells are able to put in place. The activation of repairing and detoxifying systems, as well as a reduced uptake of drugs due to cell adhesion barriers, are just a few examples of strategies in force to malignancies to protect themselves against the toxic effects of drugs. However, the most effective resistance mechanism is mainly due to the activity of the ATP-dependent efflux pumps of the ABC family, such as the P-glycoprotein (P-gp/ABCB1), multidrug resistance-associated protein 2 (MRP2/ABCC2), and the breast cancer resistance protein (BCRP/ABCG2), whose overexpression limits the prolonged and effective activity of chemotherapeutic drugs [46][37]. Additionally, in this case, aurones come in handy to obtain effective ATP-binding cassette inhibitors. Moreover, by suitably modifying the substitution pattern on either the benzylidene moiety or the benzo-fused ring, a selectivity profile against the three different efflux pumps may be also obtained. Indeed, several halogenated aurones have been described as effective P-gp inhibitors able to increase daunorubicin cyctotoxicity when tested in vitro in the chronic myelocytic leukemia K562 cell line [47][38] and paclitaxel accumulation in the human breast MDA-MB-436 cell line [48][39]. Instead, when the benzylidene moiety was replaced with an indolylmethylene residue, the resulting compound turned out to be effective against the ABCC2 protein [49][40]. Poly-methoxylated derivatives proved to interact with the ABCG2 efflux pump, inducing a mitoxantrone accumulation in cell lines [50][41]. All things considered, suitably modified members of this class of natural compounds offer the opportunity to sum up the ability to interact with key molecular anti-cancer targets with the potential to modulate the activity of efflux pumps, thus embodying the ideal anti-cancer agents.
Finally, it is worth mentioning the fluorescent potentials of this class of natural compounds. Organic molecules having fluorescence properties in the visible region of the electromagnetic spectrum are very useful investigative tools in biological systems. However, to be used for this purpose, compounds should bring only minimal perturbations to the biological macromolecules under study, to highlight their characteristics as accurately as possible to the physiological situation. Therefore, they should be characterized by rather small dimensions. Unfortunately, the currently available fluorophores, including xanthenes such as fluorescein and eosin, BODIPY, and cyanines, do not fully comply with this criterium. Shanker and co-workers demonstrated the fluorescent potential of some aurone derivatives, suggesting their possible use for biomolecular investigations [51][42]. Significantly, even the largest example proposed by the authors is smaller than xanthene dyes, and this structural characteristic is particularly advantageous for the use of the compounds. Studies on the potential application of aurone derivatives in the field of fluorescence are still ongoing but are proving to be rather promising. Ono and co-workers reported the ability of a synthetic aurone, 2-[(4-dimethylaminophenyl)methylene]-5-iodo-3(2H)-benzofuranone 24 (Figure 7), to efficiently stain Alzheimer’s mouse brain sections, thanks to the high binding affinity to the peptides of the Aβ aggregates displayed by the compound. A concomitant good brain penetration and fast washout, demonstrated through biodistribution studies carried out on normal mice, make the compound the ideal prototype probe for detecting amyloid plaques in the brain of people affected by Alzheimer disease, thus opening up a further role for these compounds [52][43].
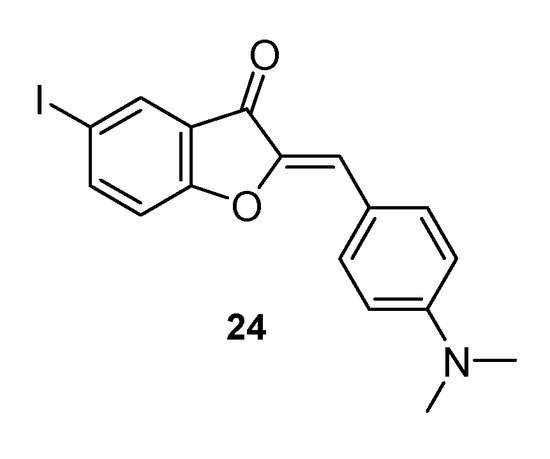
Figure 7. Synthetic aurone endowed with staining property.
The remarkable versatility of aurones, evidenced by an increasing amount of scientific evidence, makes these compounds worthy of consideration by the scientific community. However, the prospect of using them for both technological and functional purposes raises the problem of their availability. This is why, in addition to further clarifying their biological significance, it is crucial to develop synthetic strategies to obtain them in high amounts.
References
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Ann. Rev. Ecol. Evol. Syst. 2017, 48, 353–376.
- Papiorek, S.; Junker, R.R.; Alves-dos-Santos, I.; Melo, G.A.R.; Amaral-Neto, L.P.; Sazima, M.; Wolowski, M.; Freitas, L.; Lunau, K. Bees, birds and yellow flowers: Pollinator-dependant convergent evolution of UV patterns. Plant Biol. 2016, 18, 46–55.
- Davies, K.M.; Marshall, G.B.; Bradley, J.M.; Schwinn, K.E.; Bloor, S.J.; Winefield, C.S.; Martin, C.R. Characterization of aurone biosynthesis in Antirrhinum majus. Phys. Planta 2006, 128, 593–603.
- Lunau, K.; Wacht, S.; Chittka, L. Colour choices of naive bumble bees and their implications for colour perception. J. Comp. Physiol. 1996, 178, 477–489.
- Hansen, D.M.; Beer, K.; Müller, C.B. Mauritian coloured nectar no longer a mystery: A visual signal for lizard pollinators. Biol. Lett. 2006, 2, 165–168.
- Ono, E.; Fukuchi-Mizutani, M.; Nakamura, N.; Fukui, Y.; Yonekura-Sakakibara, K.; Yamaguchi, M.; Nakayama, T.; Tanaka, T.; Kusumi, T.; Tanaka, Y. Yellow flowers generated by expression of the aurone biosynthetic pathway. Proc. Natl. Acad. Sci. USA 2006, 103, 11075–11080.
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247.
- Bowry, V.W.; Ingold, K.U. The unexpected role of vitamin E (α-Tocopherol) in the peroxidation of human low-density lipoprotein. Acc. Chem. Res. 1999, 32, 27–34.
- Senthil Kimar, K.; Kumaresan, R. A Quantum chemical study on the antioxidant properties of aureusidin and bracteatin. Int. J. Quantum Chem. 2011, 111, 4483–4496.
- Olleik, H.; Yahiaoui, S.; Roulier, B.; Courvoisier-Dezord, E.; Perrier, J.; Pérès, B.; Hijazi, A.; Baydoun, E.; Raymond, J.; Boumendjel, A.; et al. Aurone derivatives as promising antibacterial agents against resistant Gram-positive pathogens. Eur. J. Med. Chem. 2019, 165, 133–141.
- Campanico, A.; Carrasco, M.P.; Njoroge, M.; Seldon, R.; Chibale, K.; Perdigão, J.; Portugal, I.; Warner, D.F.; Moreira, R.; Lopes, F. Azaaurones as potent antimycobacterial agents active against MDR- and XDR-TB. ChemMedChem 2019, 20, 1537–1546.
- Kumar, G.; Lathwal, E.; Saroha, B.; Kumar, S.; Chauhan, N.S.; Kumar, T. Synthesis and Biological Evaluation of Quinoline-Based Novel Aurones. Chem. Sel. 2020, 5, 3539–3543.
- Sutton, C.L.; Taylor, Z.E.; Farone, M.B.; Handy, S.T. Antifungal activity of substituted aurones. Bioorg. Med. Chem. Lett. 2017, 27, 901–903.
- Morimoto, M.; Fukumoto, H.; Nozoe, T.; Hagiwara, A.; Komai, K. Synthesis and insect antifeedant activity of aurones against Spodoptera litura larvae. J. Agric. Food Chem. 2007, 55, 700–705.
- Carrasco, M.P.; Newton, A.S.; Goncalves, L.; Góis, A.; Machado, M.; Gut, J.; Nogueira, F.; Hänscheid, T.; Guedes, R.C.; dos Santos, D.J.V.A.; et al. Probing the aurone scaffold against Plasmodium falciparum: Design, synthesis and antimalarial activity. Eur. J. Med. Chem. 2014, 80, 523–534.
- Ramazani, A.; Hamidnezhad, R.; Foroumadi, A.; Mirzaei, S.A.; Maddahi, S.; Hassanzadeh, S.M. In Vitro antiplasmodial activity and cytotoxic effect of (Z)-2-benzylidene-4,6-dimethoxybenzofuran-3(2H)-one derivatives. Iran J. Parasitol. 2016, 11, 371–376.
- Morimoto, M.; Cantrell, C.L.; Khan, S.; Tekwani, B.L.; Duke, S.O. Antimalarial and antileishmanial activities of phytophenolics and their synthetic analogues. Chem. Biodivers. 2017, 14, e1700324.
- Malbari, K.D.; Chintakrindi, A.S.; Ganji, L.R.; Gohil, D.J.; Kothari, S.T.; Joshi, M.W.; Kanyalkar, M.A. Structure-aided drug development of potential neuraminidase inhibitors against pandemic H1N1 exploring alternate binding mechanism. Mol. Divers. 2019, 23, 927–951.
- Zein, N.N. Clinical significance of Hepatitis C Virus genotypes. Clin. Microbiol. Rev. 2000, 13, 223–235.
- Waheed, Y.; Bhatti, A.; Ashraf, M. RNA dependent RNA polymerase of HCV: A potential target for the development of antiviral drugs. Infect. Genet. Evol. 2013, 14, 247–257.
- Meguellati, A.; Ahmed-Belkacem, A.; Yi, W.; Haudecoeur, R.; Crouillère, M.; Brillet, R.; Pawlotsky, J.-M.; Boumendjel, A.; Peuchmaur, M. B-ring modified aurones as promising allosteric inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Eur. J. Med. Chem. 2014, 80, 579–592.
- Moon, J.E.; Kim, D.-K.; Kim, J.Y. Anti-inflammatory effect of Rhus verniciflua stokes extract in the murine macrophage cell line. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 481–486.
- Sui, G.; Li, T.; Zhang, B.; Wang, R.; Hao, H.; Zhou, W. Recent advances on synthesis and biological activities of aurones. Bioorg. Med. Chem. 2021, 29, 115895–115917.
- Wang, Z.; Bac, E.J.; Han, Y.T. Synthesis and anti-inflammatory activities of novel dihydropyranoaurone derivatives. Arch. Pharm. Res. 2017, 40, 695–703.
- Park, H.S.; Nelson, D.E.; Taylor, Z.E.; Hayes, J.B.; Cunningham, K.D.; Arivett, B.A.; Ghosh, R.; Wolf, L.C.; Taylor, K.M.; Farone, M.B.; et al. Suppression of LPS-induced NF-κB activity in macrophages by the synthetic aurone, (Z)-2-((5-(hydroxymethyl) furan-2-yl)methylene) benzofuran-3(2H)-one. Int. Immunopharmacol. 2017, 43, 116–128.
- Huang, L.; Wall, M.E.; Wani, M.C.; Navarro, H.; Santisuk, T.; Reutrakul, V.; Seo, E.K.; Farnsworth, N.R.; Kinghorn, A.D. New compounds with DNA strand-scission activity from the combined leaf and stem of Uvaria hamiltonii. J. Nat. Prod. 1998, 61, 446–450.
- Suzuki, K.; Yahara, S.; Maehata, K.; Uyeda, M. Isoaurostatin, a Novel Topoisomerase Inhibitor Produced by Thermomonospora alba. J. Nat. Prod. 2001, 64, 204–207.
- Priyadarshani, G.; Nayak, A.; Amrutkar, S.M.; Das, S.; Guchhait, S.K.; Kundu, C.N.; Banerjee, U.C. Scaffold-Hopping of Aurones: 2-Arylideneimidazopyridinones as Topoisomerase IIα-Inhibiting Anticancer Agents. ACS Med. Chem. Lett. 2016, 7, 1056–1061.
- Schoepfer, J.; Fretz, H.; Chaudhuri, B.; Muller, L.; Seeber, E.; Meijer, L.; Lozach, O.; Vangrevelinghe, E.; Furet, P. Structure-based design and synthesis of 2-benzylidene-benzofuran-3-ones as flavopiridol mimics. J. Med. Chem. 2002, 45, 1741–1747.
- Dat, N.T.; Jin, X.; Hong, Y.S.; Lee, J.J. An isoaurone and other constituents from Trichosanthes kirilowii seeds inhibit hypoxia-inducible factor-1 and nuclear factor-kappa B. J. Nat. Prod. 2010, 73, 1167–1169.
- Mi, C.; Ma, J.; Shi, H.; Li, J.; Wang, F.; Lee, J.J.; Jin, X. 4′,6-dihydroxy-4-methoxyisoaurone inhibits the HIF-1a pathway through inhibition of Akt/mTOR/p70S6K/4E-BP1 phosphorylation. J. Pharmacol. Sci. 2014, 125, 193–201.
- Gerby, B.; Boumendjel, A.; Blanc, M.; Bringuier, P.P.; Champelovier, P.; Fortune, A.; Ronot, X.; Boutonnat, J. 2-Arylidenedihydroindole-3-ones: Design, synthesis, and biological activity on bladder carcinoma cell lines. Bioorg. Med. Chem. Lett. 2007, 17, 208–213.
- French, K.J.; Schrecengost, R.S.; Lee, B.D.; Zhuang, Y.; Smith, S.N.; Eberly, J.L.; Yun, J.K.; Smith, C.D. Discovery and evaluation of inhibitors of human sphingosine kinase 1. Cancer Res. 2003, 63, 5962–5969.
- Chen, H.; Qi, X.D.; Qui, P. A novel synthesis of aurones: Their in vitro anticancer activity against breast cancer cell lines and effect on cell cycle, apoptosis and mitochondrial membrane potential. Bangladesh J. Pharmacol. 2014, 9, 501–510.
- Zwick, V.; Chatzivasileiou, A.O.; Deschamps, N.; Roussaki, M.; Simões-Pires, C.A.; Nurisso, A.; Denis, I.; Blanquart, C.; Martinet, N.; Carrupt, P.A.; et al. Aurones as histone deacetylase inhibitors: Identification of key features. Bioorg. Med. Chem. Lett. 2014, 24, 5497–5501.
- Itoh, Y.; Suzuki, M.; Matsui, T.; Ota, Y.; Hui, Z.; Tsubaki, K.; Suzuki, T. False HDAC inhibition by aurone compound. Chem. Pharm. Bull. 2016, 64, 1124–1128.
- Lee, C.A.; Cook, J.A.; Reyner, E.L.; Smith, D.A. P-glycoprotein related drug interactions: Clinical importance and a consideration of disease states. Expert Opin. Drug Metab. Toxicol. 2010, 6, 603–619.
- Hadjeri, M.; Barbier, M.; Ronot, X.; Mariotte, A.M.; Boumendjel, A.; Boutonnat, J. Modulation of P-glycoprotein-mediated multidrug resistance by flavonoid derivatives and analogues. J. Med. Chem. 2003, 46, 2125–2131.
- Vaclavíkova, R.; Boumendjel, A.; Ehrlichova, M.; Kovar, J.; Gut, I. Modulation of paclitaxel transport by flavonoid derivatives in human breast cancer cells. Is there a correlation between binding affinity to NBD of P-gp and modulation of transport? Bioorg. Med. Chem. 2006, 14, 4519–4525.
- Baiceanu, E.; Nguyen, K.A.; Gonzalez-Lobato, L.; Nasr, R.; Baubichon-Cortay, H.; Loghin, F.; Le Borgne, M.; Chow, L.; Boumendjel, A.; Peuchmaur, M.; et al. 2-Indolylmethylenebenzofuranones as first effective inhibitors of ABCC2. Eur. J. Med. Chem. 2016, 122, 408–418.
- Sim, H.M.; Wu, C.P.; Ambudkar, S.V.; Go, M.L. In vitro and in vivo modulation ofABCG2 by functionalized aurones and structurally related analogs. Biochem. Pharmacol. 2011, 82, 1562–1571.
- Shanker, N.; Dilek, O.; Mukherjee, K.; McGee, D.W.; Bane, S.L. Aurones: Small molecule visible range fluorescent probes suitable for biomacromolecules. J. Fluoresc. 2011, 21, 2173.
- Ono, M.; Maya, Y.; Haratake, M.; Ito, K.; Mori, H.; Nakayama, M. Aurones serve as probes of beta-amyloid plaques in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2007, 361, 116–121.
More
