Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by MUHAMMAD WASEEM.
Drought is one of the major constraints to rain-fed agricultural production, especially under climate change conditions. Plants evolved an array of adaptive strategies that perceive stress stimuli and respond to these stress signals through specific mechanisms. Abscisic acid (ABA) is a premier signal for plants to respond to drought and plays a critical role in plant growth and development.
- ABA
- drought
- metabolites
- signaling
1. Introduction
Drought stress reduces soil water content which restricts water uptake by the plant root thereby limiting plant growth and productivity [1]. Plants have evolved a wide range of morpho-physiological, metabolic, and molecular mechanisms to resist long- or short-term responses to drought stress [2]. Phytohormones are important plant growth regulators and mediators of environmental stresses such as drought which adversely influence crop yield and pose threats to global food security [2]. To cope with drought stress, potent and novel approaches should be introduced, and phytohormone engineering could be a method of choice for sustainable crop production and breeding programs. In the last decade, the interest to understand the spatiotemporal changes of ABA to modulate plant responses is growing [3]. Abscisic acid (ABA) is critical for plant development and can redesign various physiological and biochemical signal transduction cascades in plants to cope with environmental stresses particularly drought [4,5][4][5]. Additionally, ABA plays a critical role in biomolecules biosynthesis, senescence, seed germination, stomatal closure, and root architecture modification [6,7][6][7].
ABA is classified as an isoprenoid molecule, synthesized from carotenoids (C40) derivative of isopentenyl diphosphate (IPP) through the methylerythritol phosphate (MEP) pathway in plastids [8]. The synthesis of ABA undergoes a series of steps, and each step is catalyzed by a specific enzyme. The conversion of zeaxanthin to all trans-violaxanthin is the first step in ABA biosynthesis occurring in the plastid. This cyclic hydroxylation of epoxycarotenoids to all-xanthin is catalyzed by zeaxanthin epoxidase (ZEP) through an intermediate antheraxanthin. In the next step, cis-isomerization of all trans-violaxanthin to violaxanthin or cisneoxanthin through an unknown enzymatic reaction. After that, 9-cis-epoxycarotenoid dioxygenase (NCED) enzymes split the cis-isomers of violaxanthin and neoxanthin to generate a C15 intermediate product called xanthoxin, finally exported to cytosol. In the cytosol, xanthoxin is converted into ABA through two enzymatic reactions. Next, xanthoxin is first converted to an abscisic aldehyde catalyzed by short-chain alcohol dehydrogenase/reductase (SDR). Finally, the oxidation of abscisic aldehyde to ABA by aldehyde oxidase (AAO) (Figure 1a) [9].
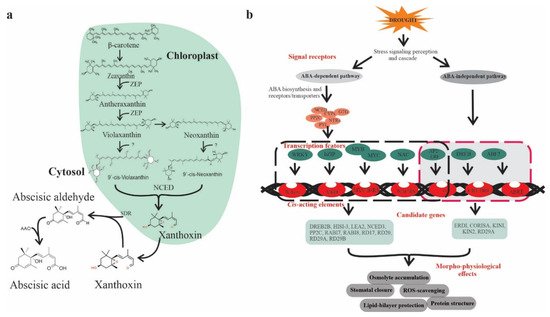
Figure 1. ABA biosynthesis and ABA-mediated drought-responsive pathways in plants. (a) Scheme of ABA biosynthesis. The precursors of ABA, β-carotene undergoes a series of oxidative reactions in the plastids and each step is catalyzed by specific enzyme such as ZEP (zeaxanthin epoxidase) or NCED (9-cis-epoxycarotenoid dioxygenase). The derived xanthoxin is exported to the cytosol and converted into ABA through an oxidation reaction mediated by AAO (aldehyde oxidase) and SDR (alcohol dehydrogenase/reductase), (b) ABA-dependent and -independent signaling pathways in the plant, which consists of several core components including ABA receptors and regulators. The ABA-dependent and -independent pathways are indicated by black and red arrows, respectively. Transcription factors (TFs) include bZIPs, MYB/MYC2, NAC (RD26), and WRKY bind to their corresponding cis-acting elements W-box, ABRE, MYB, MYC, DREB2, AREB/ABF, and NACRs.
Plants show a significant increase in ABA levels under drought stress, changes in expression of genes, and induction of ABA biosynthesis enzymes corresponding to mRNA level lead to enhanced ABA accumulation [10]. The transcript abundances of several ABA biosynthesis genes, such as ZEP/ABA1, AAO3, 9-cis-epoxycarotenoid dioxygenase (NCED3), and molybdenum cofactor sulfurase (MCSU/LOS5/ABA3), has been upregulated through an ABA-dependent or ABA-independent pathway [11] assisted by binding factors such as ABF, MYC MYB, NAC, ERF, bZIP, and DREB/CBF transcription factors (TFs) (Figure 1b) [12].
ABA is a prime mediator of drought [13] and plays an important role in regulating plant growth, development, and responses to several environmental stresses [14]. Under drought conditions, ABA-mediated stomatal conductance prevents transpiration water loss [10]. Zhang et al. [15] found that multidrug and toxic compound extrusion (MATE) transporter family, detoxification efflux carrier (AtDTX50), participate in ABA transport. ABA receptors such as PYRABACTIN RESISTANCE (PYR), or regulatory component of ABA receptor (RCAR) enhanced ABA responses and confer drought tolerance in Arabidopsis [16]. Similarly, ABA responsive-element binding protein (ABP9) a member of the bZIP family (Figure 1) improves photosynthetic capacity under drought [17]. Histone acetylation has been reported to be critical in ABA-mediated gene regulation to acclimatize plants to drought [18]. It has been shown that mitogen-activated protein kinase (MAPK) signaling cascade plays a critical role in ABA-mediated drought regulation at transcription and proteome level in various plant species including rice, maize, and Arabidopsis [19,20][19][20]. Altogether, ABA is a pivotal hormone governing plant responses to drought through complex molecular signaling mechanisms. Therefore, exploration of ABA regulators could assist in developing drought-tolerant crops through breeding programs.
2. ABA: A Key Player under Drought
Abscisic acid is of prime importance due to its stress-related responses and its involvement in various plant growth processes, making it possible to adapt to drought conditions. Upon drought stress, ABA-mediated stomatal closure reduces water loss by decreasing transpiration rate. Moreover, ABA progressively increases hydraulic conductivity and stimulates root cell elongation, enabling plants recovery from water-limited conditions [21]. Recent advancements in plant genomics accelerated the identification and functional characterization of ABA-dependent candidate genes responsive to drought. For instance, Zhang et al. [15] found that the MATE transporter gene, AtDTX50, is involved in ABA efflux, while mutants of dtx50 show enhanced tolerance to drought with reduced stomatal conductance relative to WT plants. It is widely acknowledged that ABA binds to pyrabactin-resistance 1/pyrabactin resistance like/regulatory component of aba receptor (PYR/PYL/RCAR) receptors, the initial step of the core ABA signaling pathway, concerning previously characterized protein phosphatases 2C (PP2Cs) and sucrose nonfermenting related kinases 2 (SnRK2s) (Figure 1b) [22,23,24][22][23][24]. The PYR/PYL/RCAR) proteins are reported to be involved in improving drought tolerance in many species such as Arabidopsis, tomato, and rice [25,26,27,28][25][26][27][28].
ABA has also been reported to regulate calcium-dependent protein kinases (CPK) signaling by inducing CPK6 expression under drought stress. CPKs interact and phosphorylates some core ABA-related TFs, ABFs/AREBs (ABA-responsive element-binding factors) enhancing their transcriptional activities [29]. Similarly, transgenic plants overexpressing ZEP confers tolerance to stresses such as drought [30]. Overexpression of OsbZIP72 showed increased expression of ABA-responsive gene LEAs (late embryogenesis abundant genes) and improved drought resistance in rice, which may be useful for the engineering of drought-resilient crops [31]. Arabidopsis plants overexpressing ABCG25 showed reduced water loss under drought by limiting evapotranspiration. Likewise, mutants of AtABCG40 exhibited more sensitivity to drought [32], indicating the prime importance of ABA-related genes in regulating ABA responses to drought conditions.
The ABA hormone has mainly been associated with the regulation of water deficiency in plants. A plethora of studies have shown the critical roles of ABA in regulating genes expression, proteins, and enzymatic activities involved in plant cell dehydration tolerance [33,34][33][34]. For instance, the ABA levels were exponentially elevated in Arabidopsis, wheat, rice, tomato, soybean, maize, and sesame under drought [35,36][35][36]. Similarly, Wang et al. [37] and Baek et al. [38] demonstrated how multiple genes regulate ABA-mediated drought responses in Arabidopsis, Vigna. radiata, and V. angularis. These findings suggest that ABA-mediated drought tolerance is required for plants to fully respond to drought stress.
3. ABA-Mediated Drought Responses through Physio-Biochemical Alteration
Plants have evolved distinct adaptive mechanisms to survive and minimize the adverse effect of drought stress [39]. Reactive oxygen species (ROS) serve as a signal molecule that regulates plant responses to stresses. Upon drought, plants synthesize an array of secondary metabolites (SMs) assisting plant survival [40]. ABA is able to synchronize a wide range of functions in plants, facilitating to overcome drought stress [4]. Therefore, to tackle water limitations, dynamic and novel strategies should be formulated and engineered including ROS and SMs as an adaptive strategy to maintain plant growth and productivity.
3.1. ROS Scavenging System
Drought and ABA have an intricate relationship [41] that triggers various downstream responses to plant assisting adaption to drought in an ABA-dependent manner [14]. Drought may alter the metabolic and cellular redox status of plants that influence the cellular susceptibility to ABA accumulation [42] suggested the link between metabolic status and ABA signaling [43]. ABA is an indicator of soil water deficit and endogenous ABA concentration rapidly increases to initiate stomatal closure in the plant [44]. Previous studies have also been demonstrated that drought escape induced by water stress depends on ABA. For instance, ABA could improve the plant ability to scavenge ROS by activating antioxidant enzymes [45] such as SOD (superoxide dismutase), POD (peroxidase), CAT (catalase), APX (ascorbate peroxidase), and GR (glutathione reductase) in wheat seedlings under drought, thus regulating the osmotic adjustment, reducing oxidative damage, and improving the conductivity of roots by inducing aquaporin gene expression [45,46,47][45][46][47]. Kwak et al. [48] showed that ABA activates H2O2 biosynthesis in stomata guard cells via a membrane-bound NADPH oxidase causing stomata closure by activating plasma membrane Ca2+ channels [49].
3.2. Primary Metabolism
Land plants synthesize diverse primary metabolites (PMs) having higher medicinal and nutritional value which are essential for survival [50]. In general, PMs function in protein–disulfide linkage, redox regulation, methylation reactions, including DNA methylation, mRNA capping, synthesis of phosphatidylcholine, and synthesis of polyamines [51]. Primary metabolites and their associated metabolic genes are considered pivotal factors that contribute to drought tolerance via the involvement of different metabolic pathways [52]. To date, in planta, an estimate of 200,000 metabolites are reported [53]. Among those carbohydrates, nucleosides/nucleotides, and sulfur-containing metabolites were mainly induced by ABA [54]. The major pathways responsible for PMs are glycolysis, the TCA cycle, pentose phosphate pathway, shikimate pathway, aliphatic, and aromatic amino acids which produce secondary metabolites (SMs). Abscisic acid is tightly associated with changes in water availability to fine-tune plant growth [55,56,57,58,59,60][55][56][57][58][59][60] acting as a signaling molecule for plants to adjust their metabolism and growth in response to drought stress [55].
A. thaliana and Camelina sativa ABA-inducible WSD1 (Wax synthase/acyl-CoA:diacylglycerol acyltransferase) enhanced drought tolerance through leaf and stem wax loading and epicuticular wax accumulation [61]. Canola crop is sensitive to drought, which leads to severe yield losses. However, understanding the genetic basis of ABA-mediated drought tolerance will pave the way to engineering crops with improved drought resistance. Recently, the application of Omics approaches identified various ABA-induced and suppressed proteins involved in metabolism, photosynthesis, protein synthesis, membrane transport processes, protein folding/transport and degradation, and stress/defense responsiveness [62]. This finding suggests that various ABA-induced and suppressed metabolites were used as indicators in improving our knowledge of ABA signaling to drought tolerance.
3.3. Secondary Metabolites
Plants are surrounded by a complex set of environmental stresses and respond equally to them. Plant metabolites are sensitive to changing environments such as drought [63]. The metabolic profiles of plants have been analyzed to predict their role under drought [64,65][64][65]. Plants have adapted two distinct strategies including osmotic adjustment [66] and accumulation of specialized secondary metabolites [67] to mitigate drought responses [68]. It has been shown that metabolites such as phenolic compounds, proline, glycine-betaine, soluble sugars, and other compatible solutes accumulated by plants during stress responses. These metabolites maintain water potential, cell turgor maintenance, osmotic adjustment, survival, stabilize proteins and membrane lipid bilayer structures under drought assisting to retain normal physiological processes (Figure 2) [66]. On the other hand, secondary metabolites act as scavengers of free radicals to mitigate oxidative stress in plants under drought stress.
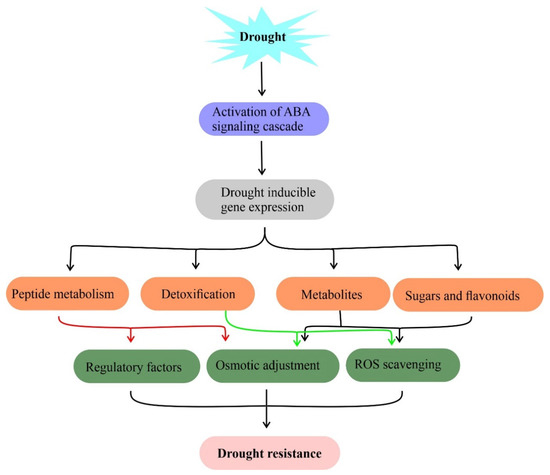

Figure 2. Metabolites and their functions in drought stress tolerance. Drought-induced accumulation of compatible solutes such as sugars, flavonoids, and amino acids for osmotic adjustment, free radical (ROS) scavenging to mitigate drought stress in plants. Genes involved in this metabolite biosynthesis against drought stress are useful in the metabolic engineering of drought resistance.
Metabolic profiling revealed ABA-inducible metabolic networks in response to drought which encourages the accumulation of dehydration-inducible branched-chain amino acids, and key dehydration-inducible genes such as lysine ketoglutarate reductase/saccharopine dehydrogenase (AtLKR/SDH), branch-chain aminotransferase (AtBCAT2), arginine decarboxylase, and delta 1-pyrroline-5-carboxylase (P5CS) [69]. For instance, the accumulation of most amino acids such as tryptophan, glutamine, alanine, proline, aspartate, leucine, isoleucine, ornithine, valine, citric acid cycle precursors including cis-aconitate, succinate, and 2-oxoglutarate; flavonoids such as cyanidin and quercetin; and lipids such as acylated sterylglycosides and glycosyl inositol phosphoceramides were increased under drought in Arabidopsis [70,71,72][70][71][72] and a few crop plants, such as maize, barley, and rice [73,74,75][73][74][75]. In maize, ZmPIS, a phosphatidylinositol synthase, efficiently improved drought tolerance by altering membrane lipid composition and ABA biosynthesis [76]. Overexpression of ABF3 in Glycine max significantly altered various primary and secondary metabolites such as glycerophospholipids, glycolipids, fatty acyls, prenol-lipids, and their derivatives [77].
References
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323.
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of Abscisic Acid in the Drought Stress Tolerance of Plants. Agronomy 2020, 10, 1323.
- Müller, M. Foes or friends: ABA and ethylene interaction under abiotic stress. Plants 2021, 10, 448.
- Wani, S.H.; Kumar, V. Plant stress tolerance: Engineering ABA: A potent phytohormone. Transcriptomics 2015, 3, 1000113.
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. FPB 2003, 30, 239–264.
- Gonzalez-Villagra, J.; Figueroa, C.; Luengo-Escobar, A.; Morales, M.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Abscisic Acid and Plant Response under Adverse Environmental Conditions. In Plant Performance under Environmental Stress; Springer: Berlin/Heidelberg, Germany, 2021; pp. 17–47.
- Trivedi, D.K.; Gill, S.S.; Tuteja, N. Abscisic acid (ABA): Biosynthesis, regulation, and role in abiotic stress tolerance. Abiotic Stress Response Plants 2016, 8, 315–326.
- Dejonghe, W.; Okamoto, M.; Cutler, S.R. Small molecule probes of ABA biosynthesis and signaling. Plant Cell Physiol. 2018, 59, 1490–1499.
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161.
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591.
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 2017, 11, 106–111.
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 1–10.
- Boominathan, P.; Shukla, R.; Kumar, A.; Manna, D.; Negi, D.; Verma, P.K.; Chattopadhyay, D. Long term transcript accumulation during the development of dehydration adaptation in Cicer arietinum. Plant Physiol. 2004, 135, 1608–1620.
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273.
- Zhang, H.; Zhu, H.; Pan, Y.; Yu, Y.; Luan, S.; Li, L. A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 2014, 7, 1522–1532.
- Saavedra, X.; Modrego, A.; Rodriݩguez, D.; Gonzaݩlez-Garciݩa, M.P.; Sanz, L.; Nicolaݩs, G.; Lorenzo, O. The nuclear interactor PYL8/RCAR3 of Fagus sylvatica FsPP2C1 is a positive regulator of abscisic acid signaling in seeds and stress. Plant Physiol. 2010, 152, 133–150.
- Zhang, X.; Wollenweber, B.; Jiang, D.; Liu, F.; Zhao, J. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J. Exp. Bot. 2008, 59, 839–848.
- Sridha, S.; Wu, K. Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. Plant J. 2006, 46, 124–133.
- Hamel, L.-P.; Nicole, M.-C.; Duplessis, S.; Ellis, B.E. Mitogen-activated protein kinase signaling in plant-interacting fungi: Distinct messages from conserved messengers. Plant Cell 2012, 24, 1327–1351.
- Muchhal, U.S.; Raghothama, K.G. Transcriptional regulation of plant phosphate transporters. Proc. Natl. Acad. Sci. USA 1999, 96, 5868–5872.
- Daszkowska-Golec, A. The role of abscisic acid in drought stress: How aba helps plants to cope with drought stress. In Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2016; Volume 2, pp. 123–151.
- Fujii, H.; Zhu, J.-K. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl. Acad. Sci. USA 2009, 106, 8380–8385.
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593.
- Vlad, F.; Rubio, S.; Rodrigues, A.; Sirichandra, C.; Belin, C.; Robert, N.; Leung, J.; Rodriguez, P.L.; Laurière, C.; Merlot, S. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 2009, 21, 3170–3184.
- González-Guzmán, M.; Rodríguez, L.; Lorenzo-Orts, L.; Pons, C.; Sarrión-Perdigones, A.; Fernández, M.A.; Peirats-Llobet, M.; Forment, J.; Moreno-Alvero, M.; Cutler, S.R. Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J. Exp. Bot. 2014, 65, 4451–4464.
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.-Y.; Yoon, I.S.; Byun, M.-O.; Kim, S.T.; Jung, K.-H.; Kim, B.-G. Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014, 65, 453–464.
- Okamoto, M.; Peterson, F.C.; Defries, A.; Park, S.-Y.; Endo, A.; Nambara, E.; Volkman, B.F.; Cutler, S.R. Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc. Natl. Acad. Sci. USA 2013, 110, 12132–12137.
- Pizzio, G.A.; Rodriguez, L.; Antoni, R.; Gonzalez-Guzman, M.; Yunta, C.; Merilo, E.; Kollist, H.; Albert, A.; Rodriguez, P.L. The PYL4 A194T mutant uncovers a key role of PYR1-LIKE4/PROTEIN PHOSPHATASE 2CA interaction for abscisic acid signaling and plant drought resistance. Plant Physiol. 2013, 163, 441–455.
- Zhang, H.; Liu, D.; Yang, B.; Liu, W.Z.; Mu, B.; Song, H.; Chen, B.; Li, Y.; Ren, D.; Deng, H.; et al. Arabidopsis CPK6 positively regulates ABA signaling and drought tolerance through phosphorylating ABA-responsive element-binding factors. J. Exp. Bot. 2020, 71, 188–203.
- Park, H.-Y.; Seok, H.-Y.; Park, B.-K.; Kim, S.-H.; Goh, C.-H.; Lee, B.-h.; Lee, C.-H.; Moon, Y.-H. Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. Biochem. Biophys. Res. Commun. 2008, 375, 80–85.
- Lu, G.; Gao, C.; Zheng, X.; Han, B. Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 2009, 229, 605–615.
- Kang, J.; Hwang, J.-U.; Lee, M.; Kim, Y.-Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360.
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54.
- Yu, Y.; Wang, P.; Bai, Y.; Wang, Y.; Wan, H.; Liu, C.; Ni, Z. The soybean F-box protein GmFBX176 regulates ABA-mediated responses to drought and salt stress. Environ. Exp. Bot. 2020, 176, 104056.
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119.
- Dossa, K.; Mmadi, M.A.; Zhou, R.; Liu, A.; Yang, Y.; Diouf, D.; You, J.; Zhang, X. Ectopic expression of the sesame MYB transcription factor SiMYB305 promotes root growth and modulates ABA-mediated tolerance to drought and salt stresses in Arabidopsis. AoB Plants 2019, 12, plz081.
- Wang, L.; Zhu, J.; Li, X.; Wang, S.; Wu, J. Salt and drought stress and ABA responses related to bZIP genes from V. radiata and V. angularis. Gene 2018, 651, 152–160.
- Baek, D.; Chun, H.J.; Kang, S.; Shin, G.; Park, S.J.; Hong, H.; Kim, C.; Kim, D.H.; Lee, S.Y.; Kim, M.C. A role for Arabidopsis miR399f in salt, drought, and ABA signaling. Mol. Cells 2016, 39, 111.
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259.
- Sreenivasulu, N.; Harshavardhan, V.T.; Govind, G.; Seiler, C.; Kohli, A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 2012, 506, 265–273.
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165.
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875.
- Verslues, P.E.; Zhu, J.K. Before and beyond ABA: Upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem. Soc. Trans. 2005, 33, 375–379.
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways. Front. Plant Sci. 2017, 8, 1493.
- Zhou, Y.; He, R.; Guo, Y.; Liu, K.; Huang, G.; Peng, C.; Liu, Y.; Zhang, M.; Li, Z.; Duan, L. A novel ABA functional analogue B2 enhances drought tolerance in wheat. Sci. Rep. 2019, 9, 2887.
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J Exp. Bot. 2004, 55, 2343–2351.
- Wu, Y.; Thorne, E.T.; Sharp, R.E.; Cosgrove, D.J. Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiol. 2001, 126, 1471–1479.
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633.
- Gilroy, S.; Read, N.; Trewavas, A.J. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature 1990, 346, 769–771.
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary–secondary metabolism interface. Curr. Opin. Biotechnol. 2011, 22, 239–244.
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509.
- Kumar, M.; Patel, M.K.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108.
- Goodacre, R.; Vaidyanathan, S.; Dunn, W.B.; Harrigan, G.G.; Kell, D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004, 22, 245–252.
- Zhu, M.; Assmann, S.M. Metabolic Signatures in Response to Abscisic Acid (ABA) Treatment in Brassica napus Guard Cells Revealed by Metabolomics. Sci. Rep. 2017, 7, 12875.
- Yoshida, T.; Obata, T.; Feil, R.; Lunn, J.E.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Fernie, A.R. The role of abscisic acid signaling in maintaining the metabolic balance required for Arabidopsis growth under nonstress conditions. Plant Cell 2019, 31, 84–105.
- Dekkers, B.J.W.; Costa, M.C.D.; Maia, J.; Bentsink, L.; Ligterink, W.; Hilhorst, H.W.M. Acquisition and loss of desiccation tolerance in seeds: From experimental model to biological relevance. Planta 2015, 241, 563–577.
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162.
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970.
- LeNoble, M.E.; Spollen, W.G.; Sharp, R.E. Maintenance of shoot growth by endogenous ABA: Genetic assessment of the involvement of ethylene suppression. J. Exp. Bot. 2004, 55, 237–245.
- Sharp, R.E.; LeNoble, M.E.; Else, M.A.; Thorne, E.T.; Gherardi, F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: Evidence for an interaction with ethylene. J. Exp. Bot. 2000, 51, 1575–1584.
- Abdullah, H.M.; Rodriguez, J.; Salacup, J.M.; Castañeda, I.S.; Schnell, D.J.; Pareek, A.; Dhankher, O.P. Increased Cuticle Waxes by Overexpression of WSD1 Improves Osmotic Stress Tolerance in Arabidopsis thaliana and Camelina sativa. Int. J. Mol. Sci. 2021, 22, 5173.
- Zhu, M.; Simons, B.; Zhu, N.; Oppenheimer, D.G.; Chen, S. Analysis of abscisic acid responsive proteins in Brassica napus guard cells by multiplexed isobaric tagging. J. Proteom. 2010, 73, 790–805.
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 1–14.
- Sweetlove, L.J.; Fell, D.; Fernie, A.R. Getting to grips with the plant metabolic network. Biochem. J. 2008, 409, 27–41.
- Fiers, M.; Golemiec, E.; Xu, J.; van der Geest, L.; Heidstra, R.; Stiekema, W.; Liu, C.-M. The 14–amino acid CLV3, CLE19, and CLE40 peptides trigger consumption of the root meristem in Arabidopsis through a CLAVATA2-dependent pathway. Plant Cell 2005, 17, 2542–2553.
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608.
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085.
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought Stress Responses and Resistance in Plants: From Cellular Responses to Long-Distance Intercellular Communication. Front. Plant Sci. 2020, 11, 556972.
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078.
- Pires, M.V.; Pereira Júnior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.M.; Florian, A.; Krahnert, I.; Maurino, V.G. The influence of alternative pathways of respiration that utilize branched-chain amino acids following water shortage in Arabidopsis. Plant Cell Environ. 2016, 39, 1304–1319.
- Tarazona, P.; Feussner, K.; Feussner, I. An enhanced plant lipidomics method based on multiplexed liquid chromatography–mass spectrometry reveals additional insights into cold-and drought-induced membrane remodeling. Plant J. 2015, 84, 621–633.
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518.
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and metabolomic studies disclose key metabolism pathways contributing to well-maintained photosynthesis under the drought and the consequent drought-tolerance in rice. Front. Plant Sci. 2016, 7, 1886.
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683.
- Chmielewska, K.; Rodziewicz, P.; Swarcewicz, B.; Sawikowska, A.; Krajewski, P.; Marczak, Ł.; Ciesiołka, D.; Kuczyńska, A.; Mikołajczak, K.; Ogrodowicz, P. Analysis of drought-induced proteomic and metabolomic changes in barley (Hordeum vulgare L.) leaves and roots unravels some aspects of biochemical mechanisms involved in drought tolerance. Front. Plant Sci. 2016, 7, 1108.
- Liu, X.; Zhai, S.; Zhao, Y.; Sun, B.; Liu, C.; Yang, A.; Zhang, J. Overexpression of the phosphatidylinositol synthase gene (ZmPIS) conferring drought stress tolerance by altering membrane lipid composition and increasing ABA synthesis in maize. Plant Cell Environ. 2013, 36, 1037–1055.
- Nam, K.-H.; Kim, H.J.; Pack, I.-S.; Kim, H.J.; Chung, Y.S.; Kim, S.Y.; Kim, C.-G. Global metabolite profiling based on GC–MS and LC–MS/MS analyses in ABF3-overexpressing soybean with enhanced drought tolerance. Appl. Biol. Chem. 2019, 62, 1–9.
More
