The cell membrane serves as a barrier and gatekeeper to regulate the cellular transportation of substances and information. It plays a significant role in protecting the cell from extracellular environment, maintaining intracellular homeostasis, and regulating the cellular function and behaviors. Capability to engineer the cell membrane with functional modules that enable dynamic monitoring and manipulating the cell-surface microenvironment would be critical for studying molecular mechanisms underlying various biological processes. To meet this goal, DNA, with intrinsic advantages of high versatility, programmability, and biocompatibility, hasve gained intense attention as a molecular tool for cell-surface engineerings as molecular tools for cell surface engineering. The past three decades have witnessed the rapid advances of diverse nucleic acid materials, including functional nucleic acids (FNAs), dynamic DNA circuits, exquisite DNA nanostructures. In this mini review, we have summarized recent progresses of DNA technology for the cell membrane engineering, particularly focused on their applications for molecular sensing and imaging, precise cell identification, receptor activity regulation and artificial membrane structures. Furthermore, we discussed the challenge and outlook on using nucleic acid materials in this specific research area.
- functional nucleic acids
- DNA nanotechnology
- cell membrane engineering
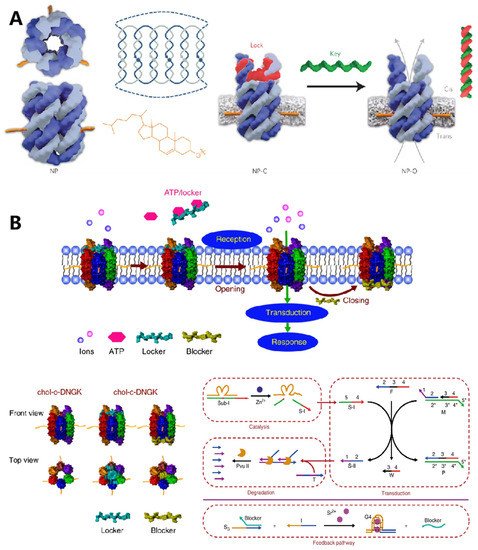
1. DNA-Based Intercellular Communication

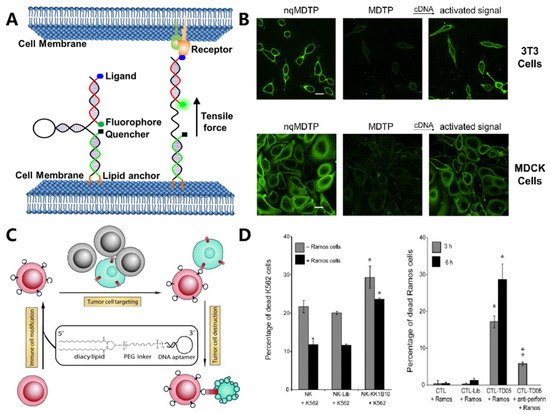
2. DNA-Based Receptor Monitoring and Regulating
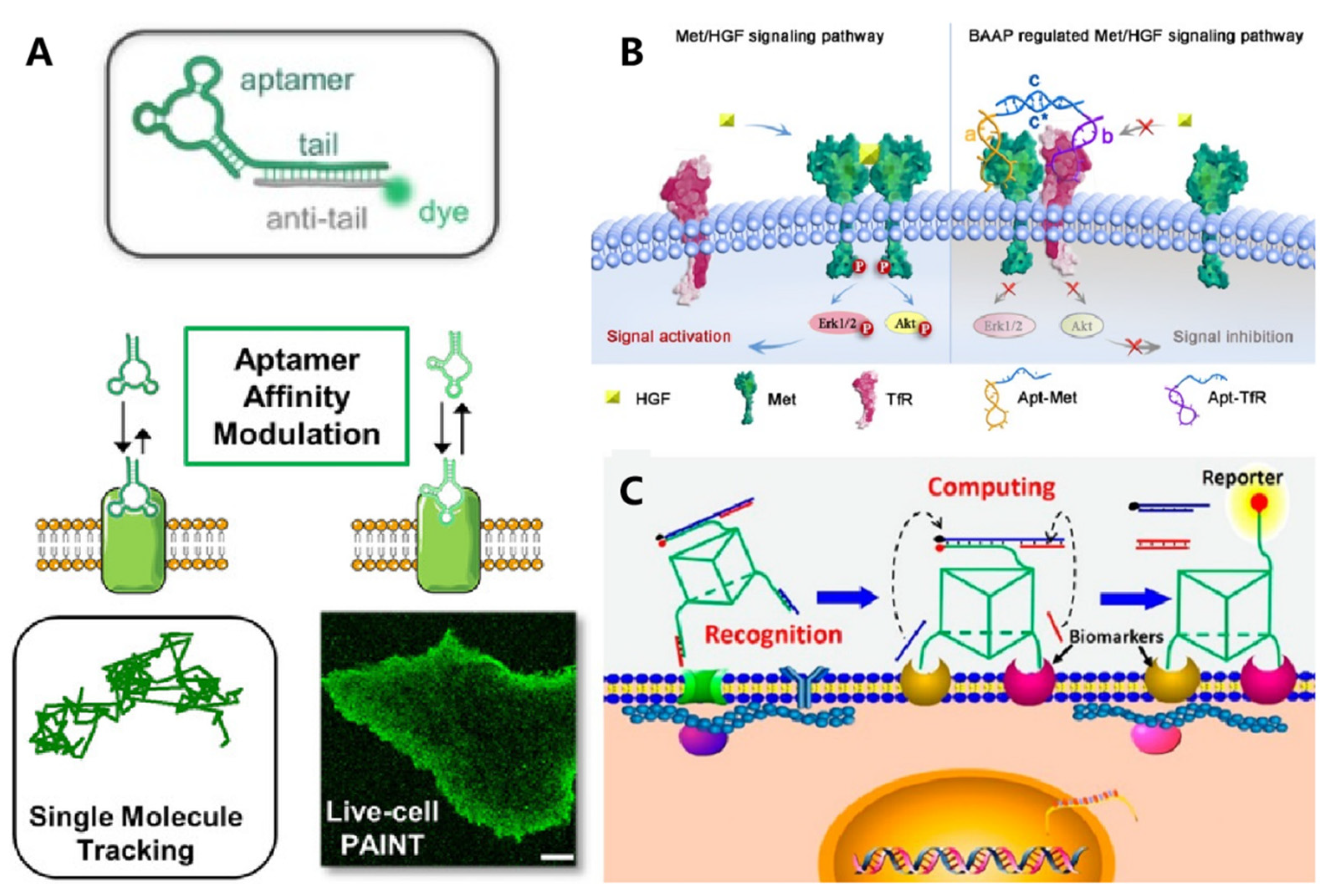
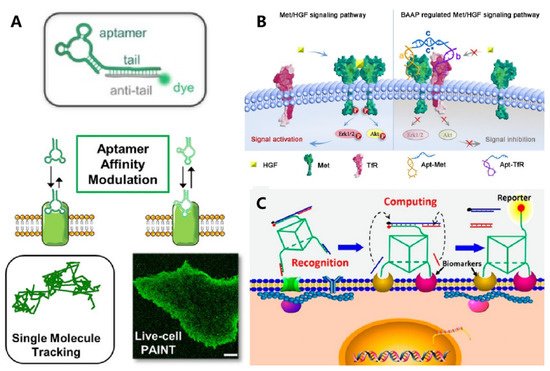
3. DNA-Based Biomimetic Membrane Constructs