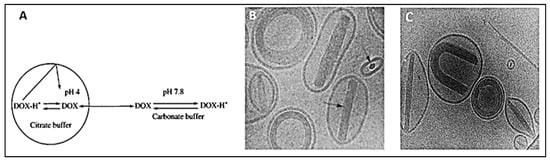
) confocal images of DOX-loaded liposomes showing the coffee-beans liposomes appearance where DOX-citrate complexes appear in rod, circular and U-shaped structures.
, Elsevier, 2001. C is adapted with permission from
, Elsevier, 1998.
Along the same lines, Haran et al.
[52][53] generated a pH gradient across the liposomes using ammonium sulfate salt. The liposomes initially encapsulated a 300 mM ammonium sulfate solution at pH 5.5 in a pH 7.4 external buffer. The higher concentration of ammonium ions on the inside led to the diffusion of the neutral ammonia molecules, and with every molecule diffusing from the core, a proton was left behind. Due to salting-out effects and the acidification of the intraliposomal compartment, high fractions of DOX can accumulate in the liposomes in an aggregated form. Liposomes loaded using this technique exhibit a prolonged stable storage period beyond 6 months because of the gelation effects of DOX with the sulfate salt, which inhibit membrane re-permeation. Alyane et al.
[53][54] examined the stability and release behavior of liposomes loaded with DOX via the ammonium sulfate transmembrane method. Liposomes comprised of hydrogenated egg yolk phosphatidylcholine (HEPS), cholesterol, and DSPE-PEG2000 at a molar ratio of 185:1:15 showed an encapsulation efficiency exceeding 90%, with a drug-to-lipid ratio of 1:20 (
w/w). Upon incubation at 37 °C for 24 h in culture media and PBS, the liposomes retained 98% and 90% of the encapsulated drug, respectively, showing minimal leakage under the stated conditions. When the incubation medium was changed to phosphate buffer at pH 5.3, the maximum release achieved was 37%. The pH responsiveness of the liposomes suggested their suitability for controlled release in acidic compartments (i.e., tumor tissues) as they would keep their cargo intact under physiological conditions. The study also revealed that encapsulating DOX in the liposomes decreased the agent’s uptake by rat myocardial H9C2 cells, suggesting the decreased cardiac toxicity of the formulation. Flow cytometry and MTT assay analysis showed that the toxic effects of DOX were reduced when compared against that of free DOX, up to 20 h of incubation time. After 20 h, the liposomal formulation became more toxic to the cells than the free one
[53][54].
DOXIL
® is a commercially available PEGylated liposomal DOX formulation that uses the ammonium sulfate gradient method to encapsulate the drug. An in vivo study by Sakakibara et al.
[54][55] investigated the performance of DOXIL
®, conventional DOX-liposomes, and free DOX on the treatment of human lung tumors. The DOXIL
® liposomes were composed of hydrogenated soy phosphatidylcholine, cholesterol, PEG-DSPE, and dl-a-tocopherol in a molar ratio of 56.1:38.2:5.5:0.2. In contrast, the non-PEGylated liposomes had phosphatidylglycerol, phosphatidylcholine, cholesterol, and DL- α -tocopherol in a molar ratio of 1:4:3:0.02. To assess and compare the different treatment groups (dosage: 1.5 mg/kg) of anti-tumor activity, human lung tumor xenografts were engrafted into the gonadal fat pad of severe combined immunodeficient (SCID) mice. The tumor eventually metastasized from the primary site into the mice’s peritoneal cavity (i.e., liver, lung). It was concluded that the PEGylated formulation successfully suppressed the primary tumor growth and arrested metastasis in the peritoneal cavity, while the free DOX was only able to stop the growth of the primary tumor without significant effects on preventing the spread. The PEGylated liposomes showed 5-folds higher circulation times than conventional liposomes, while the uptake by vital organs like the spleen was reduced. Also, a significant increase in extravasation and accumulation of the PEGylated formulation was observed, as considerable amounts were detectable at the tumors after 1 week following administration
[54][55]. Another study
[55][56] compared the in vivo pharmacokinetic performance of free DOX with PEGylated and non-PEGylated liposomal formulations. Interestingly, the PEGylated liposomal DOX clearance rate decreased by 100-folds (Cl = 0.023 L/h), and its half-life (t1/2 = 83.7 h) was prolonged by 8-folds, compared to free DOX (Cl = 25.3 L/h, t1/2 = 10.4 h). Moreover, the distribution volume decreased significantly from 364 L to 139 L to 3.0 L in the free DOX, non-PEGylated, and PEGylated liposomal DOX, respectively. This conclusion demonstrated that PEGylation prevents premature drug release and that most of it remains entrapped without leakage.
Fritze et al.
[56][57] introduced another DOX remote loading method based on a phosphate ((NH
4)
2HPO
4) transmembrane gradient, using different ammonium and sodium salts. The liposomes were composed of egg phosphatidylcholine (EPC) and cholesterol in the molar ratio 7:3. After hydrating the liposomes with 300 mM salt solutions at neutral pH, they were incubated with DOX.HCl for 12 h at 7 °C to achieve a drug-to-lipids ratio of 1:3 (mol/mol). The sizes of liposomes containing DOX dissolved in different ammonium and sodium salts, as well as their encapsulation efficiencies (EE%), are summarized in
Table 1. Results showed that loading did not significantly alter the size of the liposomes, and those loaded with the ammonium salts gradients showed higher EE than the sodium salts. Synergistic effects are suggested when using ammonium salts as they act as a reservoir for donating free protons when DOX is internalized and protonated in the acidic interior of the liposomes. Thus, intraliposomal protonation and DOX precipitation led to increased encapsulation efficiencies. Further analysis was conducted to assess the impact of varying the intravesicular ammonium concentration on liposome size and EE. DOX was dissolved in 10 mM isotonic HEPES buffered saline (HBS), 50, 100, 200, and 300 mM ammonium phosphate at pH 7.2. The sizes of the DOX-loaded liposomes were found to be 84 ± 0.4, 102 ± 1.4, 114 ± 3.6, 95 ± 1.5, and 92 ± 1.6 nm, respectively, with EE of 2.81, 23.52, 61.03, 83.35, 97.98%. Increasing the ammonium ion concentration augmented the effects of drug protonation and intravesicular acidification, which led to increased DOX diffusion across the transmembrane gradient. The least efficiency (<5%) was observed when incubating in HBS, which contains no phosphate ions (no decrease in the intraliposomal pH level), while the highest efficiency approaching 100% was observed at the highest concentration of the ammonium phosphate (300 mM).
Table 1. Summary of the size and encapsulation efficiency of loaded liposomes achieved via different salts.
| Salt Gradient |
Size ± SD (nm) |
EE (%) |
| Ammonium Phosphate |
129.3 ± 3.7 |
98 |
| Ammonium Sulfate |
129.2 ± 2.9 |
95 |
]. Benefiting from the tumor cells’ overexpression of receptors to aid in augmenting their growth and survival pathways, such receptors make promising active targets. To this end, nanocarriers can be conjugated to the ligands complementing these receptors to enhance DOX accumulation and internalization by the cancerous cells. Xing and co-workers
[62][63] examined the in vitro effects of functionalizing DOX-loaded PEGylated liposomes with a DNA aptamer (Apt-Urn liposomes) on MCF-7 cells. Flow cytometry results showed a 6.6-fold increase in drug uptake and efficacy of the functionalized loaded liposomes as opposed to the conventional ones, as the fluorescence response upon 4 h of incubation with the treatments corresponded to 93.6% and 57.0%, in the cells treated with Apt-Urn and control liposomes, respectively. Moreover, MTT assay results analyzed the cytotoxic effects of the Apt-Urn liposomes on cell viability. The cells were treated with different liposomal concentrations followed by an incubation period of 6 h, then re-cultured in fresh media, and the MTT test was done after 72 h. At a liposomal DOX concentration of 500 nM, cells treated with the functionalized liposomes exhibited a viability of 57.0 ± 6%, whereas cells treated with the control liposomes exhibited a viability of 92.4 ± 9%, evidencing the superior localization and internalization of the nanocarriers as a function of surface modification.
Yang
[63][64] conducted a recent preclinical investigation on DOX-loaded liposomes composed of EPC and cholesterol for treating head and neck cancer. The study compared three treatment groups: free DOX, DOX-loaded liposomes, and DOX-loaded peptide-conjugated liposomes. Results showed the dependence of the encapsulation efficiency on the cholesterol content in the formulations, as it increased from 20% to 79% upon the incorporation of cholesterol with EPC. Varying the EPC and cholesterol ratio yielded different entrapment efficiencies ranging from ~48% to ~82%. Both liposomal formulations, the functionalized and the conventional ones, showed sustained release profiles over a period of 40 h, but the DOX-loaded peptide-conjugated liposomes had a faster in vitro release behavior. In vivo analysis of the formulations’ efficacy was carried out on nude mice bearing HSCC (human squamous cell carcinomas) xenograft. The tumor progression was suppressed when treated with both liposomal formulations; however, the functionalized liposomes arrested metastasis and increased the median survival time of the animals in that group by 100%. The enhanced performance was due to the increased accumulation at the tumor site, resorted to localized targeting by binding to the overexpressed surface-specific receptors (Hsp47/CBP2) abundant on the head and neck cancer cells.
Another study
[64][65] examined the performance of DOX-loaded liposomes, functionalized with the monoclonal antibody Trastuzumab (TRA) for the enhanced targeting of breast cancer cells overexpressing the Human Epidermal growth factor Receptor 2 (HER2). The liposomes consisted of cholesterol, DPPC, and DSPE-PEG(2000)-NH
2 at a molar ratio of 30:65:5, respectively. DOX was loaded via the ammonium sulfate transmembrane gradient method, yielding a drug-to-lipids ratio of 1:6. The cargo release from the liposomal formulations, conventional and TRA-conjugated, was triggered using pulsed ultrasound. In vitro results showed synergistic effects on the DOX uptake and internalization upon sonication and surface modification with TRA. Similarly, Chowdhury and co-workers
[65][66] designed an in vitro study where they tested different targeted (functionalized with Aptamer-A6) and untargeted liposomal-DOX formulations for the localized treatment of HER2-overexpressing breast cancers. Twelve liposomal formulations (F1-F12) were prepared using the thin-film hydration method by varying the compositions of the phospholipid mixtures. The liposome sizes slightly increased upon DOX loading and ranged from 98.7 to 181.2 nm, with 74.9 to 94.1% entrapment efficiencies. The optimized formulations for further complexation with Aptamer-A6 were determined based on the smallest size to benefit from the EPR effect and the highest encapsulation efficiency. Formulations 5 and 8 (10 mg, 150 mg, 40 mg, 40 mg, 20 mg, and 0.25 mg of DOX, POPC, DOTAP, DOPE, DSPE-mPEG2000, and Mal-PEG, respectively) had sizes of 101.70 ± 14.04 nm and 98.7 ± 13.25 nm, respectively, and DOX entrapment efficiency of 92.8% and 94.1%, respectively. These results suggest the statistical insignificance of the hydrophobicity and charge (i.e., cationic) of the used phospholipids on the encapsulation efficiencies. However, the presence of PEG chains and the charge of the liposomal complexes directly affect their stability and integrity in long-term storage. Formulations with cationic lipids exhibited a relatively smaller size and extended shelf-life lasting up to 10 weeks, and PEG prevented liposomes from cross-linking and aggregating. Integrating Mal-PEG into the formulations aided in linking the Aptamer-A6 at its amino-terminal. In vitro analysis was done on SKBR3 and MCF7 cells, which overexpress HER2 on their surfaces. It was observed that F5 was internalized the fastest and the most by both cell lines, as flow cytometry uptake results showed the fluorescence intensities upon 2-h incubation to be 98.6% and 66.5%, respectively. SKBR3 cells uptake of F5 compared to MDA-MB-231 cells (HER2 negative cell line) was more by 1.79 times, substantiating the merit of targeted therapy.
Table 2 presents some studies involving liposomal DOX.
Table 2. Different liposomal-based DDS encapsulating DOX via the (NH4)2SO4 transmembrane gradient method.
| Preparation Method |
Target Cancer |
Functionalization |
Study Model |
Triggering Modality |
Findings |
Ref. |
| Ethanol injection |
osteosarcoma |
Estrogen |
In vitro flow cytometry and MTT analysis on MG63 (estrogen overexpressing) cells and LO2 (negative liver cells).
Ex vivo imaging of MG63 tumors extracted from Male BALB/c nude mice. |
Redox-sensitivity and glutathione responsiveness |
Loaded decorated liposomes size~110 nm.
Exhibited high encapsulation efficiency.
| Ex vivo | analysis of the functionalized liposomes showed more selective accumulation in tumor tissues compared to other vital organs, and in vitro results showed higher cytotoxicity towards overexpressing cells. |
[66] | [67] |
| Thin-film hydration |
Lymphoma |
anti-CD19 moiety; PEG grafted by disulfide links (mPEG-S-S-DSPE) |
| Ammonium Acetate |
115.9 ± 1.0 |
77 |
| Ammonium Citrate |
114.9 ± 1.2 |
100 |
| Sodium Phosphate |
113.4 ± 1.6 |
52 |
| Sodium Sulfate |
111.8 ± 1.9 |
44 |
| Sodium Acetate |
113.4 ± 1.6 |
16 |
| Sodium Citrate |
151.7 ± 3.8 |
54 |
Another study
[57][58] investigated the effect of varying the bilayer composition of DOX entrapment and release efficiency. Three liposomal formulations were tested, which included non-thermosensitive (NTS) liposomes composed of L-α-phosphatidylcholine (PC), thermosensitive (TS) liposomes composed of dipalmitoylphosphatidylcholine (DPPC) and distearoylphosphatidylcholine (DSPC), lyso-thermosensitive (LTS) liposomes composed of DPPC, DSPC, and 1-palmitoyl-2-lyso-glycero-3-phosphocholine (P-lyso-PC). All three formulations contained small amounts of cholesterol. The encapsulation efficiency of DOX dissolved in 0.9% NaCl solution was very low for TS and LTS liposomes (5.8 and 5.6%), while it was slightly higher (17.29%) for NTS liposomes because of the possibility of controlling the temperature effectively during the loading (above the phase transition temperature). In vitro drug release was modeled at controlled hyperthermia conditions (41–42 °C), and results showed that for a period of 5 h, 2%, 36%, and 54% DOX diffused from NTS, TS, and LTS liposomes. The relatively lower encapsulation efficiency and higher release performance of LTSL resort to the temperature-induced membrane instability when operating at temperatures beyond the lipids transition temperature
[57][58]. Generally, drug loading via active loading depends on several factors besides the drug’s physiochemical properties, like the extraliposomal medium condition, pH, conductivity, electrolytic activity, loading duration, as well as operating temperatures
[43][44].
Research efforts have also focused on the synergistic effects of using release triggering modalities, like ultrasound and pH, on the internalization and efficacy of DOX. Pitt et al.
[58][59] studied the in vivo performance of ultrasonically-triggered DOX-loaded liposomes on BDIX rats bearing colonic carcinoma. The liposomes were comprised of soy phosphatidyl choline, cholesterol, DSPE-PEG, and alpha-tocopherol combined in the molar ratio 3:1:1:0.004, and DOX was entrapped via the ammonium sulfate gradient method. Using sonication remarkably enhanced the DOX release kinetics and effects on mice. The group treated with liposomes followed by ultrasound exposure (20 kHz for 15 min, once for 4 weeks) showed significant regression in tumors growth to an immeasurable size by the end of the treatment period. Likewise, another in vivo study
[59][60] on mice bearing SCC7 murine squamous carcinoma cells showed that sonication using pulsed high-frequency US (HFUS) enhanced the performance of the proposed liposomal drug delivery system. Fluorescent spectrophotometry results proved that the mean DOX concentration in the sonicated tumors was 124% more than in the control, which received the liposomal treatment but without sonication. Work by Zhang et al.
[60][61] synthesized pH-sensitive liposomes by modifying their surfaces via the insertion of poly(2-ethyl-2-oxazoline)-cholesteryl methyl carbonate (PEOZ-CHMC) copolymer. The performance of these liposomes was compared to others grafted with PEG-DSPE chains. DOX hydrochloride was loaded into both types of liposomes by the ammonium sulfate transmembrane gradient method. It was revealed that functionalization with PEOZ-CHMC and PEG-DSPE had no effects either on the drug’s encapsulation efficiency, which was 97.3 ± 1.4, or on the size, which was ~120 nm. In vitro release done via dialysis showed that the release profile from PEOZ-CHMC-DOX liposomes in PBS at pH 5.0 significantly surpassed that observed at pH 7.4, suggesting a strong relationship between medium acidity and cargo release from the modified liposomes. Moreover, MTT assay results proved a direct relationship between the antiproliferative effects of the PEOZ-CHMC-DOX liposomes and pH conditions. The pH-sensitive formulation showed higher activity inhibition to the MCF-7 cells at lower pH; herein, pH can be used as a triggering mechanism for drug release.
As mentioned, scheming highly efficient liposomal drug delivery systems with complete dependence on passive targeting is hampered by limitations like the possible accumulation of the nanocarriers in the spleen and liver as these organs have fenestrated vasculature and their incapability to sufficiently penetrate deep enough through the complex tumoral network due to heterogeneities in structure
[61][62
| In vitro MTT assay |
| In vivo model: Female BALB/c Cr Alt B/M mice bearing Namalwa cells |
pH sensitivity |
Liposomes decorated with cleavable PEG chains rapidly dissociated in the plasma. The pH-sensitive liposomes, targeting the CD19 epitope excessively abundant on B-lymphoma cells, |
| showed increased selective cytotoxicity towards these cells, and enhanced release kinetics at lower pH levels. |
[67] | [68] |
| Post-insertion; mixing with preformed DOXIL |
Cancer Stem Cells (CSCs) |
anti-CD44 monoclonal antibody (mAb) |
In vitro flow cytometry and MTT assay on C-26 and NIH-3T3 (non-tumor) cells.
In vivo model: female BALB/c mice bearing C-26 colon carcinoma. |
N/A |
Functionalization of DOXIL liposomes significantly increased their size. The IC | 50 | values were lower on the C-26 cell line overexpressing CD44, while higher values were reported for the negative cell line (NIH-3T3). |
[68] | [69] |
| Solvent evaporation |
Various cancers |
Cationic Polymethacrylate Eudragit RL100 |
In vitro flow cytometry and MTT assay on MCF7/adr and H22 cells.
In vivo model: ICR mice bearing aggressive liver cancer H22 cells. |
N/A |
Functionalization of liposomes with Polymethacrylate derivatives increases their cellular internalization and antitumoral activity. The in vivo results showed that four injections of the functionalized formulation led to tumor size reduction by 60%. |
[69] | [70] |
| Thin-film hydration |
Metastatic lung cancer |
CXCR4-antagonist cyclic peptide (peptide R) |
In vitro cytotoxicity assay.
In vivo model: C57BL/6 mice bearing B16 human melanoma cells |
N/A |
In vitro results showed that targeting significantly decreased the IC | 50 | while reducing metastasis and regression in tumor size growth. |
[70] | [71] |
| Film dispersion |
hepatocellular carcinoma (HCC) |
glycyrrhetinic acid (GA) and peanut agglutinin (PNA) |
In vitro specific uptake of HepG2, MCF-7, and SMMC-7721 cells
In vivo model: male BALB/C-nu mice bearing SMMC-7721 xenografts. |
N/A |
HepG2 cells showed the highest uptake towards the liposomes functionalized with GA alone, while MCF-7 showed the highest affinity towards the PNA functionalized liposomes. The dual-targeted liposomal formulation was most internalized by the SMMC-7721 |
[71] | [72] |
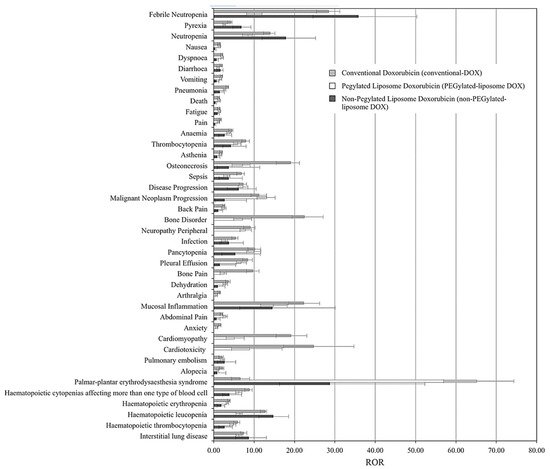
As many liposomal DOX formulations have successfully translated to clinical applications, and others are still in the clinical testing phase, it is essential to compare their performance and overall pharmacovigilance against the free drug.
Table 3 presents a comparison between phase III clinical trials results of two current commercially available liposomal DOX formulations, namely Myocet
® and DOXIL
®, against conventional DOX. Clinical studies have shown that DOXIL
® reduced the constraints on the dose limits of free DOX administration, in addition to reducing the cardiotoxic and myocardial damage incidences even at high doses exceeding the 500 mg/m
2 threshold
[72][73][73,74]. Fukuda et al.
[74][75] presented the first large-scale study that compared the adverse effects (AEs) associated with treatments of conventional, PEGylated (DOXIL
®, Caelyx
®, and LipoDox
®), and non-PEGylated (Myocet
®) liposomal DOX formulations, based on data collected from 7,561,254 patient reports (from 2004 to 2015) retrieved from the Food and Drug Administration Adverse Event Reporting System (FAERS). The analysis was based on the top 30 AEs correlated with conventional DOX treatments, including nausea, diarrhea, vomiting, anemia, cardiomyopathy, and cardiotoxicity.
Figure 7 summarizes the findings, which show the reporting odds ratios (RORs) of each treatment for each AE. ROR is a pharmacovigilance index that reflects the chances of occurrence, detection, and prevention of AEs of drug formulations. The lower the ROR, the more patient-friendly the formulation is. As reported, all DOX treatments have been correlated with severities of myelosuppression, cardiotoxicity, alopecia, nausea, and vomiting; however, both liposomal DOX formulations show relatively better safety profiles than conventional DOX.