Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Elena Bogdanova and Version 2 by Vivi Li.
The unique nutrition of the Arctic Indigenous Peoples is associated with their increased endurance, health, and adaptability to the harsh climate. Reindeer meat, blood, and liver are the most critical elements of this traditional nutrition enriched with minerals. Reindeer consumption is a crucial factor of successful adaptation to the cold stress, as well as a component of national culture, food, and economic security and sovereignty, affecting the well-being and health of the Indigenous population in the Arctic.
- reindeer meat
- macro- and microelement analysis
- adaptation
- Arctic population
- meta-analysis
1. Introduction
The unique nutrition of the Arctic Indigenous Peoples is associated with their increased endurance, health, and adaptability to the harsh climate [1]. Reindeer meat, blood, and liver are the most critical elements of this traditional nutrition enriched with minerals [2][3][2,3]. Reindeer consumption is a crucial factor of successful adaptation to the cold stress, as well as a component of national culture, food, and economic security and sovereignty, affecting the well-being and health of the Indigenous population in the Arctic [4][5][6][7][8][9][4,5,6,7,8,9].
The reindeer (Rangifer tarandus) habitat covers territories in Eurasia and North America between 50- and 81-degrees north latitude [10] and includes continental and island territories, tundra, taiga, and mountainous areas close to them in vegetation composition and climatic conditions [11]. Reindeer live in Russia, the USA, Norway, Sweden, Finland, Denmark, Iceland, Canada, Mongolia, Great Britain, and China [10]. The largest populations of wild reindeer (Rangifer tarandus caribou) are in Russia (952.9 thousand; 2015) and Canada (1300 thousand; 2016) [11]. The world’s largest livestock of domesticated reindeer is in Russia (1620.8 thousand reindeer in 2021) [12]. In Russia, the largest population of wild reindeer is in the Krasnoyarsky Krai, the Chukotka Autonomous Okrug, the Republic of Sakha (Yakutia), and domesticated reindeer are in the Yamal-Nenets Autonomous Okrug [13]. Such various reindeer habitats make pre-conditions for the different chemical compositions of reindeer products in different northern regions.
The macro- and microelement composition of reindeer meat is impacted by significant differences in the species and mineral composition of forages (plants and lichens), the duration of grazing seasons on winter and summer pastures, the proportion in the diet of green fodder, shrubs, lichens, mushrooms, eggs of birds, and rodents, the macro- and microelement composition of soil and water, pollution, availability of salty seawater, and the cutting of velvet antlers [14][15][14,15]. A specific feature of the northern reindeer is its seasonal migration to areas with different forage resources: Summer pastures with a predominance of herbaceous plants and shrubs and winter pastures rich in lichens [16].
The study of the macro- and microelement composition of reindeer meat started in the second half of the 20th century. In the 1970s, in Canada, O. Schaefer (1977) and K. Hoppner (1978) confirmed the high nutritional value of reindeer meat due to high protein and low fat content [17][18][17,18]. Two decades later, H.V. Kuhnlein (1992; 1996; 2000; 2002) conducted a study of micronutrient composition of reindeer products [19][20][21][22][19,20,21,22] and developed recommendations for the use of venison by patients with atherosclerosis, vitamin deficiency, diabetes mellitus, and for the prevention of heart, liver, and stomach diseases [23][24][25][23,24,25]. In the 1990s, in Alaska, the USA, the chemical composition of traditional products, including venison, was studied [26]. Currently, a national database includes the data on the complete quantitative and qualitative chemical composition of reindeer meat in Alaska [27]. In Russia, studies conducted in Yamal-Nenets Autonomous Okrug [28][29][28,29], Nenets Autonomous Okrug [30][31][32][30,31,32], Taimyr [33][34][35][33,34,35], the Republic of Yakutia [36], and on the Kola Peninsula [37][38][39][37,38,39] confirmed the nutritional and biological value of reindeer meat. Furthermore, they proved the need to include this product in a healthy diet.
Rangifer tarandus is highly adapted to Arctic conditions. The optimal work of enzymes that ensure adaptation to cold stress provides the accumulation of essential trace elements necessary for the practical work of enzymatic chains. The most crucial macronutrients are calcium (Ca), magnesium (Mg), phosphorus (P), potassium (K), and sodium (Na), among others, which activate enzymes, regulate the number of hormones, promote muscle and nervous activity, and therefore are essential components of the daily human diet [40][41][42][40,41,42]. Thus, the consumption of reindeer meat can increase adaptation to the Arctic conditions, reduce the risk of heart diseases, and improve metabolism [43][44][45][43,44,45].
Improving knowledge about the macro- and microelement composition of reindeer meat in different northern regions will contribute to the expansion of the use of reindeer products to prevent diseases and increase the adaptation of the Arctic population and shift workers in the circumpolar area, as well as develop effective medicinal and pharmaceutical products. Furthermore, studying the chemical composition of reindeer meat will also increase the value of exported reindeer meat, which is an important factor in promoting the economic sovereignty and well-being of the Indigenous Peoples in the Arctic.
2. Macro- and Microelement Composition in Reindeer Meat: Heterogeneity Analysis
2.1. Magnesium
The iron content in reindeer meat was available in 11 studies. The standardised mean differences ranged from 2.9107 to 11.0987; most ratings were positive (100%). The estimated standardised mean difference based on a random-effects model was 5.3972 (95% CI: 3.7340–7.0604). Thus, the mean value was significantly different from zero (z = 6.3602, p < 0.0001) (Table 12, Figure 12).
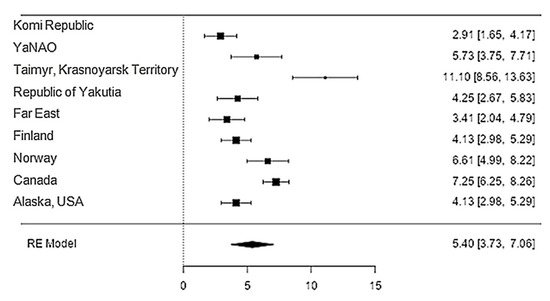

Figure 12. Forest plot of the comparison of the content of magnesium in reindeer (Rangifer tarandus) meat by geographical regions.
Table 12.
The content of macro- and microelements in reindeer (Rangifer tarandus) meat: Heterogeneity analysis.
| Macro- and Microelements |
Random-Effects Model, k | Estimate * | se | Z | p | CI Lower | CI Upper |
| Magnesium | 9 | 5.40 | 0.849 | 6.36 | <0.001 | 3.734 | 7.060 |
| Iron | 9 | 5.83 | 1.31 | 4.43 | <0.001 | 3.250 | 8.404 |
| Zinc | 9 | 0.51 | 0.149 | 3.45 | <0.001 | 0.22 | 0.804 |
| Calcium | 9 | −2.12 | 2.45 | −0.867 | 0.386 | −6.918 | 2.674 |
| Potassium | 10 | 24.3 | 25.4 | 0.96 | 0.34 | −25.45 | 73.99 |
| Sodium | 9 | 24.1 | 23.7 | 1.02 | 0.31 | −22.31 | 70.5 |
| Phosphorus | 9 | 14.5 | 19.0 | 0.76 | 0.45 | −22.7 | 51.7 |
| Heterogeneity Statistics | |||||||
| Macro- and Microelements |
Tau | Tau² | I² | H² | df | Q | p |
| Magnesium | 2.419 | 5.8524 (SE = 3.259) |
92.17% | 12.776 | 8.000 | 66.719 | <0.001 |
| Iron | 3.832 | 14.686 (SE = 7.81) |
97.04% | 33.77 | 8.000 | 269.34 | <0.001 |
| Zinc | 0.44 | 0.194 (SE = 0.0995) |
97.67% | 42.98 | 8.000 | 429.42 | <0.001 |
| Calcium | 7.292 | 53.1782 (SE = 26.9478) |
99.3% | 142.905 | 8.000 | 488.351 | <0.001 |
| Potassium | 79.87 | 6378.95 (SE = 3034.51) |
99.44% | 178.16 | 9.000 | 1970.58 | <0.001 |
| Sodium | 71.00 | 5041.41 (SE = 2522.57) |
99.94% | 1779.06 | 8.000 | 8955.84 | <0.001 |
| Phosphorus | 56.8 | 3227.16 (SE = 1621.7) |
99.54% | 216.18 | 8.000 | 2146.4 | <0.001 |
* Note. Tau² Estimator: Hedges.
) meat: Heterogeneity analysis.
The Q-test confirmed the heterogeneity of the sources, including the data on the content of magnesium in reindeer (Rangifer tarandus) meat (Q(8) = 66.72, p < 0.0001, tau2 = 5.85, I2 = 92.17%). The 95% interval was from 0.37 to 10.42. Publication bias was explored with a visual inspection of the funnel plot (Figure 23), where the regression test showed asymmetry in the funnel plot (p = 0.026), but not the rank correlation test (p = 0.3429) (Table 23).
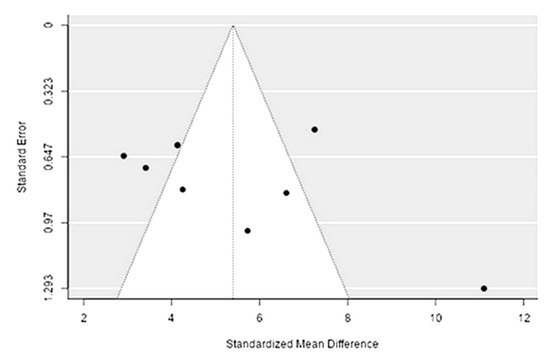

Figure 23. Funnel plot for publication bias evaluation of magnesium content in reindeer (Rangifer tarandus) meat by geographical regions.
Table 23.
The statistical analysis of publication bias of the included sources with the data on macro- and microelements content in reindeer (Rangifer tarandus) meat *.
| Macro- and Microelements |
Test | |||
|---|---|---|---|---|
| Fail-Safe N | Egger’s Regression | |||
| Value | p | Value | p | |
| Magnesium | 1559.000 | <0 .001 | 2.221 | 0.026 |
| Iron | 1284.000 | <0 .001 | 3.33 | 0.001 |
| Zinc | 1689.000 | <0 .001 | −0.099 | 0.921 |
| Calcium | 226.0 | <0 .001 | −0.14 | 0.89 |
| Potassium | 735.0 | <0 .001 | −0.14 | 0.89 |
| Sodium | 735.0 | <0 .001 | −0.14 | 0.89 |
| Phosphorus | 225.0 | <0 .001 | 1.3 | 0.19 |
* Fault-tolerant calculation of N using Rosenthal’s approach.
) meat *.
2.2. Iron
The iron content in reindeer meat was available in 11 studies. The standardised mean differences ranged from 0.32 to 11.56, and most ratings were positive (100%). The estimated standardised mean difference was 5.83 (95% CI: 3.25–8.4) based on a random-effects model. Thus, the mean value was significantly different from zero (z = 4.43, p < 0.0001) (Table 12, Figure 34).
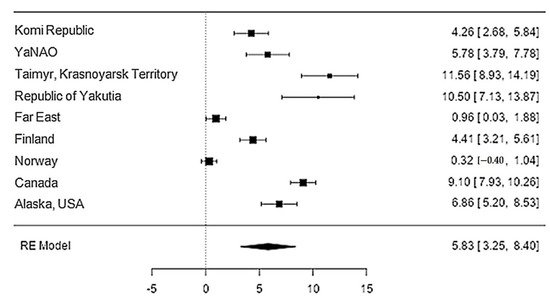

Figure 34. Forest plot of the sources, including the data on the iron content in reindeer (Rangifer tarandus) meat in different geographical regions.
The Q-test confirmed the heterogeneity of the sources, including the data on the content of iron in reindeer (Rangifer tarandus) meat (Q(8) = 269.34, p < 0.0001, tau2 = 14.69, I2 = 97.04%). The 95% interval was from −2.11 to 13.77. Publication bias was explored with a visual inspection of the funnel plot (Figure 45), where the regression test showed asymmetry in the funnel plot (p = 0.0009), but not the rank correlation test (p = 0.12) (Table 23).
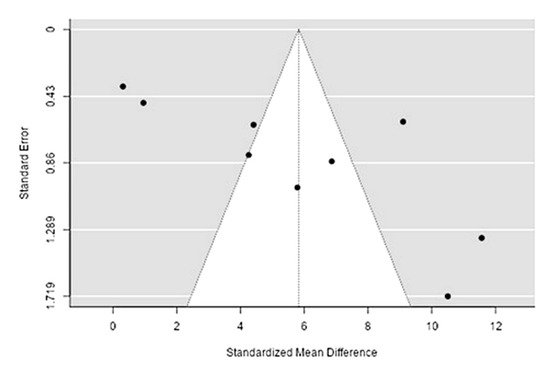

Figure 45. Funnel plot of the sources, including the data on the content of iron in reindeer (Rangifer tarandus) meat in different geographical regions.
2.3. Zinc
Data on the content of zinc in reindeer meat were available in 11 studies. The standardised mean differences ranged from −0.05 to 1.52, with most ratings being positive (89%). The estimated standardised mean difference based on a random-effects model was 0.51 (95% CI: 0.22–0.80). Thus, the mean value was significantly different from zero (z = 3.45, p < 0.0006) (Table 12, Figure 56).
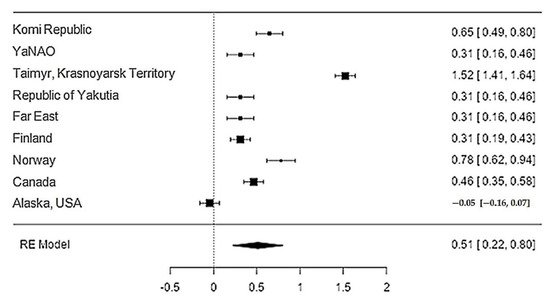

Figure 56. Forest plot of the sources, including the data on the content of zinc in reindeer (Rangifer tarandus) meat in different geographical regions.
The Q-test confirmed the heterogeneity of the sources, including the data on the content of zinc in reindeer (Rangifer tarandus) meat (Q(8) = 429.42, p < 0.0001, tau2 = 0.194, I2 = 97.67%). The 95% interval was from −0.399 to 1.42. Publication bias was explored with a visual inspection of the funnel plot (Figure 67), where the rank correlation and regression tests were p = 0.45 and p = 0.92, respectively (Table 23).
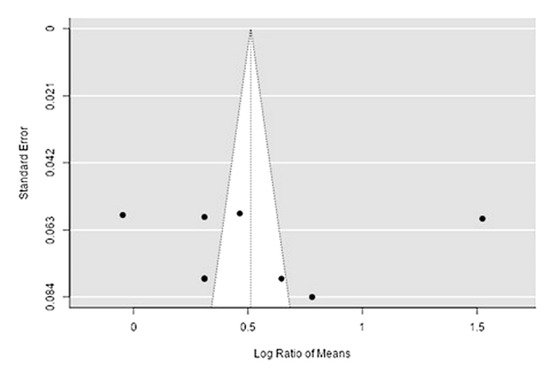

Figure 67. Funnel plot of the sources, including the data on the content of zinc in reindeer (Rangifer tarandus) meat in different geographical regions.
2.4. Calcium
Data on calcium content in reindeer meat were available in 11 studies. The standardised mean differences ranged from −14.9 to 7.2, with most ratings being negative (56%). The estimated standardised mean difference was −2.1 (95% CI: −6.92–2.67) based on a random-effects model. Thus, the mean value was significantly different from zero (z = −0.87, p = 0.39) (Table 12, Figure 78).
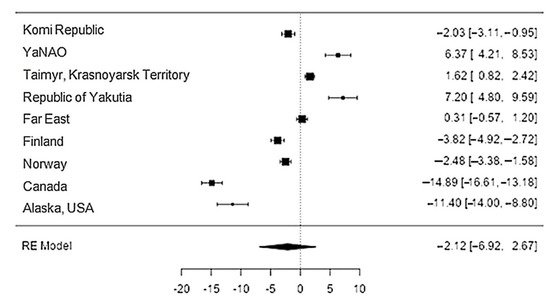

Figure 78. The forest plot of the sources includes the data on the calcium content in reindeer (Rangifer tarandus) meat in different geographical regions.
The Q-test confirmed the heterogeneity of the sources, including the data on the content of calcium in reindeer (Rangifer tarandus) meat (Q(8) = 488.35, p < 0.0001, tau2 = 53.18, I2 = 99.3%). The 95% interval was from −17.2 to 12.96. Publication bias was explored with a visual inspection of the funnel plot (Figure 89), where the rank correlation and regression test did not reveal any asymmetry in the funnel plot (p = 0.26 and p = 0.89, respectively) (Table 23).
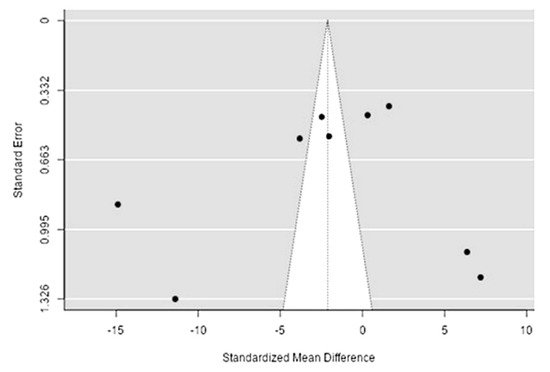

Figure 89. The funnel plot of the sources includes the data on the calcium content in reindeer (Rangifer tarandus) meat in different geographical regions.
2.5. Potassium
Data on potassium content in reindeer meat were available in 11 studies. The standardised mean differences ranged from −25.45 to 73.99, with most ratings being negative (70%). The estimated standardised mean difference was 24.3 (95% CI: −25.45–73.99) based on a random-effects model. Thus, the mean value was significantly different from zero (z = 0.96, p = 0.34) (Table 12, Figure 910).
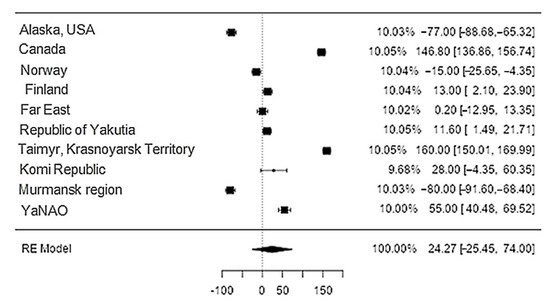

Figure 910. Forest plot of the sources, including the data on potassium content in reindeer (Rangifer tarandus) meat in different geographical regions.
The Q-test confirmed the heterogeneity of the sources, including the data on the content of potassium in reindeer (Rangifer tarandus) meat (Q(9) = 1970.58, p < 0.0001, tau2 = 6378.65, I2 = 99.44%). The 95% interval was from −164.4 to 161.95. Publication bias was explored with a visual inspection of the funnel plot (Figure 110), where the rank correlation and regression tests were p = 0.48 and p = 0.88, respectively (Table 23).
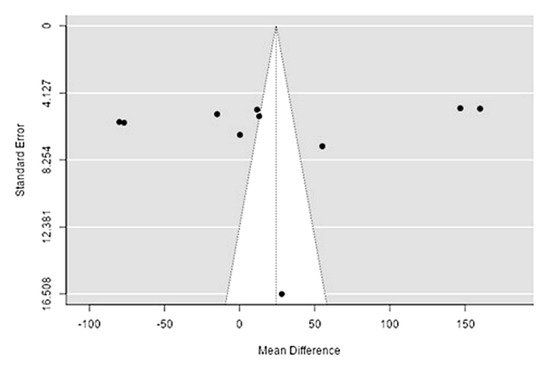

Figure 110. Funnel plot of the sources, including the data on potassium content in reindeer (Rangifer tarandus) meat in different geographical regions.
2.6. Sodium
Data on the content of sodium in reindeer meat were available in 11 studies. The standardised mean differences ranged from −27.7 to 198.6, with most ratings being negative (44%). The estimated standardised mean difference was 24.1 (95% CI: 22.31–70.5) based on a random-effects model. Thus, the mean value was significantly different from zero (z = 1.02, p = 0.31) (Table 12, Figure 112).
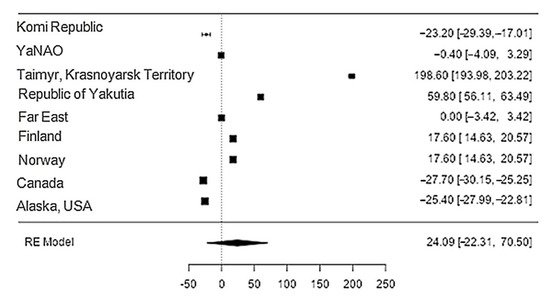

Figure 112. Forest plot of the sources, including the data on sodium content in reindeer (Rangifer tarandus) meat in different geographical regions.
The Q-test confirmed the heterogeneity of the sources, including the data on the content of sodium in reindeer (Rangifer tarandus) meat (Q(8) = 8955.85, p < 0.0001, tau2 = 5041.41, I2 = 99.94%). The 95% interval was from −122.6 to 170.8. Publication bias was explored with a visual inspection of the funnel plot (Figure 123), where the rank correlation and regression tests were p = 0.14 and p = 0.46, respectively (Table 23).
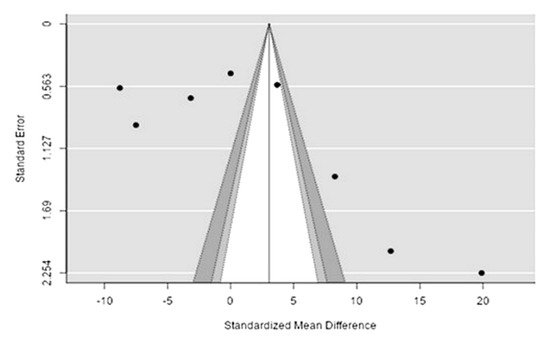

Figure 123. Funnel plot of the sources, including the data on sodium content in reindeer (Rangifer tarandus) meat in different geographical regions.
2.7. Phosphorus
The data on phosphorus content in reindeer meat was available in 11 studies. The standardised mean differences ranged from −27.7 to 198.6, with most ratings being positive (78%). The estimated standardised mean difference was 14.5 (95% CI: −22.7 to 51.7) based on a random-effects model. Thus, the mean value was significantly different from zero (z = 0.763, p = 0.45) (Table 12, Figure 134).
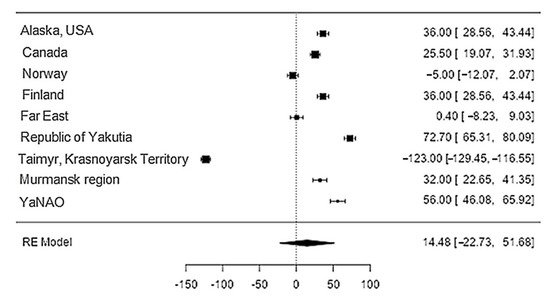

Figure 134. Forest plot of the sources, including the data on phosphorus content in reindeer (Rangifer tarandus) meat in different geographical regions.
The Q-test confirmed the heterogeneity of the sources, including the data on the content of phosphorus in reindeer (Rangifer tarandus) meat (Q(8) = 2146.4, p < 0.0001, tau2 = 3227.16, I2 = 99.54%). The 95% interval was from −102.9 to 131.9. Publication bias was explored with a visual inspection of the funnel plot (Figure 145), which did not present significant asymmetry: The rank correlation and regression tests were p = 0.34 and p = 0.19, respectively (Table 23).
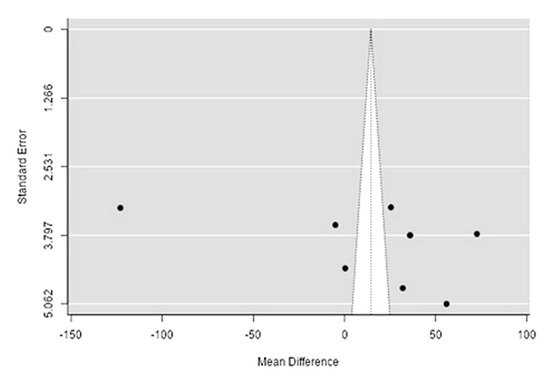

Figure 145. Funnel plot of the sources, including the data on phosphorus content in reindeer (Rangifer tarandus) meat in different geographical regions.
