Vertebrate cutaneous sensory corpuscles are specialized sensory nerve formations located in the skin of all vertebrates and responsible for tactile sensation. Functionally, they are mechanoreceptors transducing external mechanical stimuli into electrical signals which will be later led to the Central Nervous System.
The afferent innervation of vertebrate skin is supplied by nerve fibers (Aβ, Aδ, C) which are originated from peripheral neurons localized in the dorsal root ganglia (DRG). Aβ nerve fibers end at the dermis level forming several morphotypes of sensory corpuscles with capacity of detecting different stimuli: Merkel cell–neurite complexes, Ruffini corpuscles, Meissner’s corpuscles and Pacinian corpuscles are present in the glabrous skin; while pilo-neural complexes are found in hairy skin.
The structure of sensory corpuscles is formed by an axon, non-myelinating Schwann-like cells, a capsule of endoneurial and/or perineurial origin and extracelullar matrix molecules.
The vertebrate skin contains sensory corpuscles that are receptors for different qualities of mechanosensitivity like light brush, touch, pressure, stretch or vibration. These specialized sensory organs are linked anatomically and functionally to mechanosensory neurons, which function as low-threshold mechanoreceptors connected to peripheral skin through Aβ nerve fibers. Furthermore, low-threshold mechanoreceptors associated with Aδ and C nerve fibers have been identified in hairy skin. The process of mechanotransduction requires the conversion of a mechanical stimulus into electrical signals (action potentials) through the activation of mechanosensible ion channels present both in the axon and the periaxonal cells of sensory corpuscles (i.e., Schwann-, endoneurial- and perineurial-related cells). Most of those putative ion channels belong to the degenerin/epithelial sodium channel (especially the family of acid-sensing ion channels), the transient receptor potential channel superfamilies, and the Piezo family.
- sensory corpuscles
- mechanoreceptor.
- Meissner corpuscle
- Pacinian corpuscle
- Ruffini corpuscle
- Merkel cell-neurite complex
- peripheral nervous system
The vertebrate skin contains sensory corpuscles that are receptors for different qualities of mechanosensitivity like light brush, touch, pressure, stretch or vibration. These specialized sensory organs are linked anatomically and functionally to mechanosensory neurons, which function as low-threshold mechanoreceptors connected to peripheral skin through Aβ nerve fibers. Furthermore, low-threshold mechanoreceptors associated with Aδ and C nerve fibers have been identified in hairy skin. The process of mechanotransduction requires the conversion of a mechanical stimulus into electrical signals (action potentials) through the activation of mechanosensible ion channels present both in the axon and the periaxonal cells of sensory corpuscles (i.e., Schwann-, endoneurial- and perineurial-related cells). Most of those putative ion channels belong to the degenerin/epithelial sodium channel (especially the family of acid-sensing ion channels), the transient receptor potential channel superfamilies, and the Piezo family.
1. Introduction
- Introduction
Tactile sensation is one of the most important components of mechanosensation, and originates in nerve fibers that can be distinguished based on the morphology of their skin terminals (i.e., free nerve endings and sensory corpuscles), as well as on the conduction speed of their action potentials. The sensory corpuscles are the receptors responsible for tactile modalities including light brush, touch, pressure sensation, stretch, and vibration [1–3][1][2][3]. These mechanosensitivity modalities depend on Aβ, Aδ and C nerve fibers (distinguished according to axon diameter, degree of myelination, and axonal conduction velocity) connected to low-threshold mechanoreceptors (LTMRs).
LTMR sensory neurons are pseudo-unipolar, and the axonal processes that extend to the skin are associated with specialized cells: Merkel cells (forming Merkel cell–neurite complexes), Schwann-like cells that form part of the sensory corpuscles (Meissner corpuscles, Ruffini’s corpuscles, Pacinian corpuscles), or cells of hair follicles (sensory nerve endings associated to hair follicles) [3–6][3][4][5][6]. Aβ fibers originate in intermediate- or large-sized mechanosensory neurons and are the main fiber type mediating discriminative touch [4[4][7][8],7,8], although Aδ fibers [9] [9]and C fibers [10] from small-sized neurons are also involved in mechanosensation.
Mechanotransduction is defined as the conversion of mechanical stimuli into electrical signals, and this process occurs at the periphery of LTMRs, inside the sensory corpuscles [11–13][11][12][13]; in this context, the sense of touch is a prime example of mechanotransduction in biology [14–16][14][15][16]. Each morphotype of sensory corpuscle is assumed to detect different qualities of touch. Therefore, understanding mechanotransduction in free nerve endings and sensory corpuscles requires the identification of the various molecular mechanisms that translate cell-tissue deformation into action potential firing in the corresponding LTMR.
Classically, both the mechanical properties of periaxional cells of the sensory corpuscles and differentiations on the axonal membrane were considered necessary and sufficient to generate the so-called receptor potential and consequently the action potential (see for review [3,6,17][17]). Nevertheless, the discovery that certain ion channels are at the basis of sensitivity, and that mechanical forces can trigger some ion channels (mechanosensitive or mechanically gated ion channels) support that mechanotransduction occurs via those ion channels. Consistently, it is believed that LTMRs innervating the skin [18–24] [18][19][20][21][22][23][24] and their cutaneous target cells [25] display ion channels activated by force or displacement. In other words, gating of ion channels present in cutaneous sensory corpuscles in response to mechanical stimuli is the first step to transduce mechanical energy into electrical activity [18,20,23,26][26].
- Glabrous Skin
2. Glabrous Skin
The body surface of mammals is covered by two structural types of skin, i.e., non-hairy, or glabrous, and hairy skin. Glabrous skin contains no hairs, has a thick epidermal layer, and is restricted to zones characterized by high discriminative touch (shape, size, texture) as the palm of hands or the plant of foot. Hairy skin covers more than 90% of the body surface, has a thin epidermal layer and is strongly associated with affective touch [3].
In the glabrous skin, four types of terminals associated with LTMRs have been functionally identified, each associated with different cells or sensory corpuscles, all of which have more or less specific tuning properties: Merkel cell–neurite complexes, Ruffini corpuscles, Meissner’s corpuscles and Pacinian corpuscles [2–6,27] ([27]Figures 1 and 2).
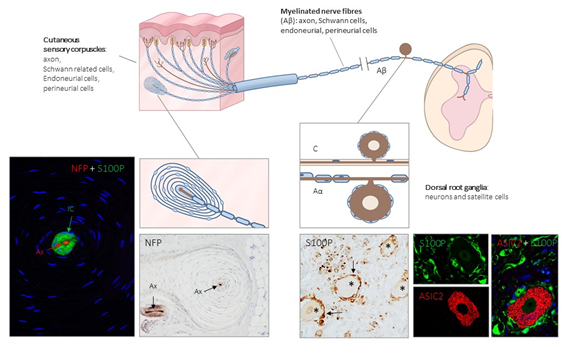
Figure 1. Schematic representation of the afferent innervation of mammalian glabrous skin. Glabrous skin is supplied by myelinated and non-myelinated nerve fibers (Aβ, Aδ, C), originated from large, intermediate and small sized neurons (low-threshold mechanoreceptors (LTMRs) and nociceptors) localized in the dorsal root ganglia (DRG). Aβ nerve fibers end in the dermis forming different morphotypes of sensory corpuscles. Photos on the left side correspond to sections of Pacini’s corpuscles immunostained for neurofilament proteins (NFP) and S100 protein (S100P) to, respectively, label the axon (Ax; red immunofluorescence) and the Schwann-related cells (IC: inner core; green fluorescence). Right side photos correspond to a section of human lumbar DRG—immunostained for S100P and acid-sensing ion channel protein 2 (ASIC2). S100 protein labels satellite cells (arrows and green fluorescence) while neuronal cell bodies (asterisks and red fluorescence) display ASIC2 positivity.
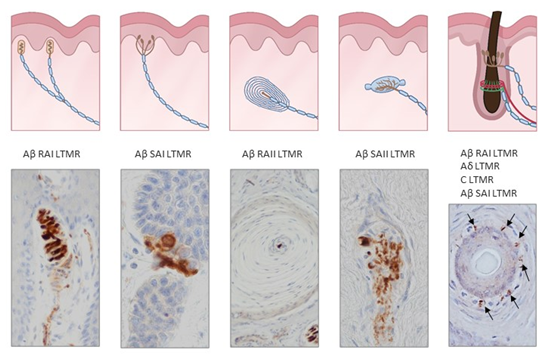
Figure 2. Schematic representations and photos of the different sensory corpuscle morphotypes present in human digital glabrous and hairy skin. Aβ LTMRs contact with epithelial Merkel cells or Schwann-like cells forming Merkel cell–neurite complexes (Aβ slowly adapting (SA)I-LTMRs), Meissner corpuscles (Aβ rapidly adapting (RA)1-LTMRs), Pacinian corpuscles (Aβ RAII-LTMRs) and Ruffini endings (Aβ SAII-LTMRs). Hairs have a complex nervous apparatus that consists of lanceolate and circumferential endings as well as free nerve endings; occasionally, hairs have associated Merkel cells, Ruffini and even Pacinian corpuscles. Photos were obtained from sections of human digital and facial skin immunostained for neuron-specific enolase to label the central axon, i.e., the ending of Aβ low-threshold mechanoreceptors.
Structurally, cutaneous sensory corpuscles consist of a central axon, surrounded by non-myelinating Schwann-like cells variably arranged, and a capsule of endoneurial and/or perineurial derivation [6,17,28,29][28][29][30][31]. It must be emphasized that although the term central axon is widely used to denominate the neuronal component of sensory corpuscles, it actually represents the peripheral process of a pseudo-unipolar neuron, localized in dorsal root ganglia (DRG) or the sensory ganglia of the cranial nerves. Therefore, the so-called central axon corresponds sensu stricto to a dendrite, or better a dendritic zone as denominated classically (see [6,30]). Filling the spaces among the cells there is a chemically complex extracellular matrix [31–33][32][33][34].
Cutaneous sensory corpuscles represent differentiated morphotypes of the Aβ LTMRs end organs. They fall functionally into two main categories: rapidly adapting (RA) and slowly adapting (SA) mechanoreceptors, which each can be sub-divided into two variants, type I and type II [3,27]. SAI mechanoreceptors are associated with epidermal Merkel cell–neurite complexes and are tuned by both static and dynamic stimuli. SAII mechanoreceptors are thought to be located in dermal Ruffini’s corpuscles although other sensory corpuscles are presumed to function as SAII [34][35] and are particularly sensitive to stretch. RAI and RAII mechanoreceptors are Meissner’s and Pacinian sensory corpuscles, respectively; Meissner’s corpuscles detect movement across the skin, and Pacinian corpuscles respond to vibrations [3,27].
2.1. Merkel Cell–Axon Complexes
Merkel cells are specialized epidermal cells [35] [31] functionally connected to Aβ SAI-LTMRs to accomplish tactile discrimination of shapes and textures [36,37][36][37]. They are present in glabrous skin especially in touch-sensitive areas, such as fingertips and lips, as well as in specialized spots in hairy skin called touch domes [38,39][38][39].
The Merkel cell–neurite complex consists of two distinct but closely associated cell types: Aβ sensory neurons and the specialized epithelial cells denominated Merkel cells. The contacts between epithelial Merkel cells and the afferent terminals are synaptic-like ones (see [37]) that use glutamate [40[40][41],41], adrenalin [42], or serotonin [43] as a neurotransmitter. Recently we also found indirect evidence for an ATP-mediated neurotransmission (L. Cárcaba et al., unpublished). They also express ion channels directly related to or required for mechanotransduction (see below).
2.2. Meissner’s Corpuscles
Meissner corpuscles are Aβ RAI-LTMRs sensitive to dynamic skin deformation, but that resolve spatial detail poorly [27,34]. They are specific to human and primate glabrous skin, and are located within the dermal papillae, concentrated in areas particularly sensitive to light touch like fingertips, palms, soles, lips, face and the skin of male and female genitalia. Meissner’s corpuscles’ size and morphology are varied, but often they present an ellipsoid morphology being 80–150 μm in length and 20–40 μm in diameter [5,6]. They consist of an axon from an Aβ nerve fiber, non-myelinating lamellar Schwann-related cells, and a more or less developed capsule of endoneurial origin [29,44][44].
2.3. Pacini Corpuscles
Cutaneous Pacinian corpuscles are structurally complex specialized sensory formations localized in hypodermis, that work as Aβ RAII-LTMRs connected to Aβ sensory nerve fibers [12,13]. They are oblong shaped, usually about 1 mm in length and display a typical onion-like structure. They consist of a central axon, sheated by non-myelinating Schwann-like cells forming the so-called inner core (with proper specific arrangements at the corpuscular terminal and ultraterminal segments) [30], both surrounded by the so-called intermediate layer of endoneurial cells, and all covered by the outer core–capsule complex of perineurial cells arranged in a multilayered concentric fashion [6,28,45][45].
2.4. Ruffini’s Corpuscles
Little information is available about cutaneous Ruffini’s corpuscles [4,5]. SAII-LTMRs have been extensively characterised physiologically [46,47][46][47] but not morphologically. In many cases, SAII-LTMR responses have been recorded in nerve fibres innervating a tissue [46], but no evidence of Ruffini corpuscles in such tissues was morphologically present [48–50][48][49][50]. Cutaneous Ruffini’s corpuscles are fusiform structures with tapered ends. They consist of a single axon with numerous terminal branches embedded in a core of Schwann-related cells and collagen, all surrounded by a multilayered capsule of perineurial origin [4,5,13]. Functionally they mediate stretching information [27,34].
- Hairy Skin: The Pilo–Neural Complexes
3. Hairy Skin: The Pilo–Neural Complexes
In the hairy skin of mammals, three major types of hairs are found: guard hairs, awl/auchene hairs, and zigzag hairs, which are densely innervated by functionally distinct sensory nerve fibers: Aβ innervates guard hairs; Aδ- (or D-hair receptors) and C-fibers, respectively, innervate awl/auchene and zigzag hairs. The peripheral endings of those nerve fibers are arranged as palisades (lanceolate endings), or as collars or rings (circumferential endings) [3]. In addition, Merkel cells and rarely Pacinian corpuscles are also present in the hairy skin associated to the follicles. Aβ SAI-LTMRs and Merkel cells form complexes to detect skin indentation denominated touch domes [51].
The hair follicle shaft is innervated by lanceolate and circumferential endings belonging to Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs; lanceolate endings are mainly sensitive to movement and low-frequency vibration [52,53][52][53]. The neck of hair follicles contains unmyelinated free nerve ending LTMRs (Figures 2 and 3). In mammals other than humans, D-hair receptors are the most sensitive mechanoreceptor of hairy skin, and there is practically no evidence for their existence in human hairy skin [54].
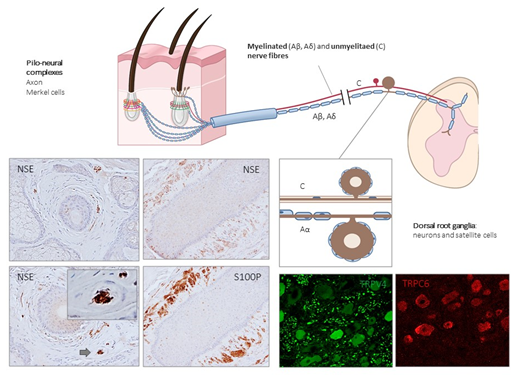
Figure 3. Schematic representation of the afferent innervation of mammalian hairy skin. Hairs form pilo–neural complexes with Aβ, Aδ, and C nerve fibers originated from large, intermediate and small sized primary sensory neurons localized in dorsal root ganglia (DRG). They form circumferential and longitudinal lanceolate endings around the hair follicle that work as RA-LTMRs, Aδ-LTMRs, and C-LTMRs. Guard hairs are innervated by Aβ RA-LTMR lanceolate endings; awl/auchene hairs by Aβ RA-LTMRs, Aδ-LTMRs and C-LTMRs lanceolate endings; and zigzag hairs by Aδ-LTMRs and C-LTMRs. Merkel cell touch domes are innervated by Aβ SAI-LTMRs. NSE: neuron-specific enolase; S100P: S100 protein; TRPC6: transient receptor potential canonical channel 6; TRPV4: transient-receptor potential vanilloid channel 4.
A population of unmyelinated LTMRs axons, so-called C-LTMRs, also innervate the hairy skin. The existence of C-LTMRs has been known for many decades, but is currently ignored, although they are relatively common in human skin [55]. The function of C-LTMRs is still unknown, and has been related to pleasant sensations, often associated with touch [56], and could play a role in mechanical hypersensitivity after nerve or tissue injury [57][57].
Thus, the distinct sensory functions of glabrous and hairy skin are not only defined by their neurophysiological aspects, but also have noticeable morphological differences.
References
- Barr-Gillespie, P.G.; Walker, R.G. Molecular basis of mechanosensory transduction. Natural 2001, 413, 194–202, doi:10.1038/35093011.
- McGlone, F.; Reilly, D. The cutaneous sensory system. Neurosci. Biobehav. Rev. 2010, 34, 148–159, doi:10.1016/j.neubiorev.2009.08.004.
- Zimmerman, A.; Bai, L.; Ginty, D.D. The gentle touch receptors of mammalian skin. Science 2014, 346, 950–954, doi:10.1126/science.1254229.
- Rice, F.; Albrecht, P. Cutaneous Mechanisms of Tactile Perception: Morphological and Chemical Organization of the Innervation to the Skin. In The Senses: A Comprehensive Reference; Elsevier BV: Amsterdam, The Netherlands, 2008; Volume 6, pp. 1–31.
- Munger, B.L.; Idez, C. The structure and function of cutaneous sensory receptors. Arch. Histol. Cytol. 1988, 51, 1–34, doi:10.1679/aohc.51.1.
- Zelená, J. Nerves and Mechanoreceptors; Chapman & Hall: London, UK, 1994.
- Gardner, E.P.; Martin, J.H.; Jessell, T.M. The bodily senses. In Principles of Neural Science, 4th ed.; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds.; McGraw-Hill: New York, USA, 2000; pp. 430–449.
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639, doi:10.1016/j.neuron.2013.07.051.
- Djouhri, L. Aδ-fiber low threshold mechanoreceptors innervating mammalian hairy skin: A review of their receptive, electrophysiological and cytochemical properties in relation to Aδ-fiber high threshold mechanoreceptors. Neurosci. Biobehav. Rev. 2016, 61, 225–238, doi:10.1016/j.neubiorev.2015.12.009.
- Djouhri, L. Electrophysiological evidence for the existence of a rare population of C-fiber low threshold mechanoreceptive (C-LTM) neurons in glabrous skin of the rat hindpaw. Neurosci. Lett. 2016, 613, 25–29, doi:10.1016/j.neulet.2015.12.040.
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The Functional Organization of Cutaneous Low-Threshold Mechanosensory Neurons. Cell 2011, 147, 1615–1627, doi:10.1016/j.cell.2011.11.027.
- Roudaut, Y.; Lonigro, A.; Coste, B.; Hao, J.; Delmas, P.; Crest, M. Touch sense. Channels 2012, 6, 234–245, doi:10.4161/chan.22213.
- Fleming, M.S.; Luo, W. The anatomy, function, and development of mammalian Aβ low-threshold mechanoreceptors. Front. Biol. 2013, 8, 408–420, doi:10.1007/s11515-013-1271-1.
- Chalfie, M. Neurosensory mechanotransduction. Nat. Rev. Mol. Cell Biol. 2009, 10, 44–52, doi:10.1038/nrm2595.
- Hoffman, B.D.; Grashoff, C.; A. Schwartz, M. Dynamic molecular processes mediate cellular mechanotransduction. Natural 2011, 475, 316–323, doi:10.1038/nature10316.
- Schneider, E.R.; Gracheva, E.O.; Bagriantsev, S.N. Evolutionary Specialization of Tactile Perception in Vertebrates. Physiology 2016, 31, 193–200, doi:10.1152/physiol.00036.2015.
- Vega, J.; García‐Suárez, O.; Montaño, J.A.; Pardo, B.; Cobo, J.M. The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microsc. Res. Tech. 2009, 72, 299–309, doi:10.1002/jemt.20651.
- Lumpkin, E.A.; Caterina, M.J. Mechanisms of sensory transduction in the skin. Natural 2007, 445, 858–865, doi:10.1038/nature05662.
- Tsunozaki, M.; Bautista, D.M. Mammalian somatosensory mechanotransduction. Curr. Opin. Neurobiol. 2009, 19, 362–369, doi:10.1016/j.conb.2009.07.008.
- Lumpkin, E.A.; Marshall, K.L.; Nelson, A.M. The cell biology of touch. J. Cell Biol. 2010, 191, 237–248, doi:10.1083/jcb.201006074.
- Gu, Y.; Gu, C. Physiological and pathological functions of mechanosensitive ion channels. Mol. Neurobiol. 2014, 50, 339–347, doi:10.1007/s12035-014-8654-4.
- Paluch, E.K.; Nelson, C.M.; Biais, N.; Fabry, B.; Moeller, J.; Pruitt, B.; Wollnik, C.; Kudryasheva, G.; Rehfeldt, F.; Federle, W. Mechanotransduction: Use the force(s). BMC Biol. 2015, 13, 47, doi:10.1186/s12915-015-0150-4.
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179, doi:10.1016/j.neuron.2015.08.032.
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, Tension, and Transduction—The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci. 2016, 42, 57–71, doi:10.1016/j.tibs.2016.09.004.
- Cobo, R.; García-Mesa, Y.; García-Piqueras, J.; Feito, J.; Martín-Cruces, J.; García-Suárez, O.; Vega, J. The Glial Cell of Human Cutaneous Sensory Corpuscles: Origin, Characterization, and Putative Roles. In Somatosensory and Motor Research; IntechOpen: London, UK, 2020.
- Delmas, P.; Coste, B. Mechano-Gated Ion Channels in Sensory Systems. Cell 2013, 155, 278–284, doi:10.1016/j.cell.2013.09.026.
- Jones, L.A.; Smith, A.M. Tactile sensory system: Encoding from the periphery to the cortex. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 279–287, doi:10.1002/wsbm.1267.
- García-Piqueras, J.; García-Suárez, O.; Rodríguez-González, M.; Cobo, J.; Cabo, R.; Vega, J.; Feito, J. Endoneurial-CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann. Anat.-Anat. Anz. 2017, 211, 55–60, doi:10.1016/j.aanat.2017.01.006.
- García‐Piqueras, J.; Cobo, R.; Cárcaba, L.; García‐Mesa, Y.; Feito, J.; Cobo, J.; García‐Suárez, O.; Vega, J. The capsule of human Meissner corpuscles: Immunohistochemical evidence. J. Anat. 2019, 236, 854–861, doi:10.1111/joa.13139.
- Malinovský, L. Sensory nerve formations in the skin and their classification. Microsc. Res. Tech. 1996, 34, 283–301, doi:10.1002/(sici)1097-0029(19960701)34:43.0.co;2-q.
- Van Keymeulen, A.; Mascre, G.; Youseff, K.K.; Harel, I.; Michaux, C.; De Geest, N.; Szpalski, C.; Achouri, Y.; Bloch, W.; Hassan, B.A.; et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 2009, 187, 91–100, doi:10.1083/jcb.200907080.
- García-Piqueras, J.; García-Suárez, O.; García-Mesa, Y.; García-Fernández, B.; Quirós, L.M.; Cabo, R.; Martín-Biedma, B.; Feito, J.; Vega, J.A. Heparan sulfate in human cutaneous Meissner's and Pacinian corpuscles.. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2020, 303, 2262–2273, 10.1002/ar.24328
- García-Piqueras, J.; Carcaba, L.; García-Mesa, Y.; Feito, J.; García, B.; Viña, E.; Suárez-Quintanilla, J.; Cobo, J.; Vega, J.; García-Suárez, O. Chondroitin Sulfate in Human Cutaneous Meissner and Pacinian Sensory Corpuscles. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2018, 302, 325–331, doi:10.1002/ar.23951.
- García-Piqueras, J.; García-Mesa, Y.; Feito, J.; García, B.; Quiros, L.; Martín-Biedma, B.; Cobo, T.; Vega, J.; García-Suárez, O. Class I and Class II small leucine-rich proteoglycans in human cutaneous pacinian corpuscles. Ann. Anat.-Anat. Anz. 2019, 224, 62–72, doi:10.1016/j.aanat.2019.02.007.
- Olson, W.; Dong, P.; Fleming, M.; Luo, W. The specification and wiring of mammalian cutaneous low‐threshold mechanoreceptors. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 389–404, doi:10.1002/wdev.229.
- Iggo, A.; Muir, A.R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J. Physiol. 1969, 200, 763–796, doi:10.1113/jphysiol.1969.sp008721.
- Maksimovic, S.; Baba, Y.; Lumpkin, E.A. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann. N. Y. Acad. Sci. 2013, 1279, 13–21, doi:10.1111/nyas.12057.
- Lacour, J.; Dubois, D.; Pisani, A.; Ortonne, J. Anatomical mapping of Merkel cells in normal human adult epidermis. Br. J. Dermatol. 1991, 125, 535–542, doi:10.1111/j.1365-2133.1991.tb14790.x.
- Boot, P.M.; Rowden, G.; Walsh, N. The Distribution of Merkel Cells in Human Fetal and Adult Skin. Am. J. Dermatopathol. 1992, 14, 391–396, doi:10.1097/00000372-199210000-00003.
- Fagan, B.M.; Cahusac, P.M. Evidence for glutamate receptor mediated transmission at mechanoreceptors in the skin. NeuroReport 2001, 12, 341–347, doi:10.1097/00001756-200102120-00032.
- Hitchcock, I.S.; Genever, P.G.; Cahusac, P.M. Essential components for a glutamatergic synapse between Merkel cell and nerve terminal in rats. Neurosci. Lett. 2004, 362, 196–199, doi:10.1016/j.neulet.2004.02.071.
- Hoffman, B.U.; Baba, Y.; Griffith, T.N.; Mosharov, E.V.; Woo, S.-H.; Roybal, D.D.; Karsenty, G.; Patapoutian, A.; Sulzer, D.; A. Lumpkin, E. Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses. Neuron 2018, 100, 1401–1413.e6, doi:10.1016/j.neuron.2018.10.034.
- Chang, W.; Kanda, H.; Ikeda, R.; Ling, J.; Gu, J.G. Serotonergic transmission at Merkel discs: Modulation by exogenously applied chemical messengers and involvement of Ih currents. J. Neurochem. 2017, 141, 565–576, doi:10.1111/jnc.14009.
- Vega, J.A.; López-Muñiz, A.; Calavia, M.G.; García-Suarez, O.; Cobo, J.; Otero, J.; Arias-Carrion, O.; Perez-Pinera, P.; Menéndez-González, M. Clinical Implication of Meissner’s Corpuscles. CNS Neurol. Disord.-Drug Targets 2012, 11, 856–868, doi:10.2174/1871527311201070856.
- Bell, J.; Bolanowski, S.; Holmes, M.H. The structure and function of pacinian corpuscles: A review. Prog. Neurobiol. 1994, 42, 79–128, doi:10.1016/0301-0082(94)90022-1.
- Johansson, R.S.; Vallbo, A.B. Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979, 286, 283–300, doi:10.1113/jphysiol.1979.sp012619.
- Wellnitz, S.A.; Lesniak, D.R.; Gerling, G.J.; Lumpkin, E.A. The Regularity of Sustained Firing Reveals Two Populations of Slowly Adapting Touch Receptors in Mouse Hairy Skin. J. Neurophysiol. 2010, 103, 3378–3388, doi:10.1152/jn.00810.2009.
- Rice, F.L.; Rasmusson, D.D. Innervation of the digit on the forepaw of the raccoon. J. Comp. Neurol. 2000, 417, 467–490, doi:10.1002/(sici)1096-9861(20000221)417:43.0.co;2-q.
- Paré, M.; Smith, A.M.; Rice, F.L. Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. J. Comp. Neurol. 2002, 445, 347–359, doi:10.1002/cne.10196.
- Paré, M.; Behets, C.; Cornu, O. Paucity of presumptive ruffini corpuscles in the index finger pad of humans. J. Comp. Neurol. 2003, 456, 260–266, doi:10.1002/cne.10519.
- Woodbury, C.J.; Koerber, H.R. Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. J. Comp. Neurol. 2007, 505, 547–561, doi:10.1002/cne.21517.
- Konietzny, F.; Hensel, H. Response of rapidly and slowly adapting mechanoreceptors and vibratory sensitivity in human hairy skin. Pflüg. Arch.-Eur. J. Physiol. 1977, 368, 39–44, doi:10.1007/bf01063452.
- Heidenreich, M.; Lechner, S.G.; Vardanyan, V.; Wetzel, C.; Cremers, C.W.; De Leenheer, E.M.; Aránguez, G.; Moreno-Pelayo, M.A.; Jentsch, T.J.; Lewin, G.R. KCNQ4 K+ channels tune mechanoreceptors for normal touch sensation in mouse and man. Nat. Neurosci. 2011, 15, 138–145, doi:10.1038/nn.2985.
- Adriaensen, H.; Gybels, J.; O. Handwerker, H.; Van Hees, J. Response properties of thin myelinated (A-delta) fibers in human skin nerves. J. Neurophysiol. 1983, 49, 111–122, doi:10.1152/jn.1983.49.1.111.
- Olausson, H.W.; Wessberg, J.; Morrison, I.; McGlone, F.; Vallbo, Åke The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010, 34, 185–191, doi:10.1016/j.neubiorev.2008.09.011.
- Löken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547–548, doi:10.1038/nn.2312.
- Seal, R.P.; Wang, X.; Guan, Y.; Raja, S.N.; Woodbury, C.J.; Basbaum, A.I.; Edwards, R.H. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Natural 2009, 462, 651–655, doi:10.1038/nature08505.