Early successful conception of postpartum dairy cows is crucial in determining the optimum reproductive efficiency and profitability in modern dairy farming. Due to the inherent high production potential of modern dairy cows, the extra stress burden of peri-parturient events, and associated endocrine and metabolic changes causes negative energy balance (NEBAL) in postpartum cows. The occurrence of NEBAL is associated with excessive fat mobilization in the form of non-esterified fatty acids (NEFAs). The phenomenon of NEFA mobilization furthers with occurrence of ketosis and fatty liver in postpartum dairy cows. High NEFAs and ketones are negatively associated with health and reproductive processes. An additional burden of hypocalcemia, ruminal acidosis, and high protein metabolism in postpartum cows presents further consequences for health and reproductive performance of postpartum dairy cows.
- dairy cow
- parturition
- fertility
- metabolic disorders
- reproductive performance
- ketosis
- milk fever
- fatty liver
1. Introduction
2. Nutritional Characteristics, Metabolic Diseases, and Reproduction
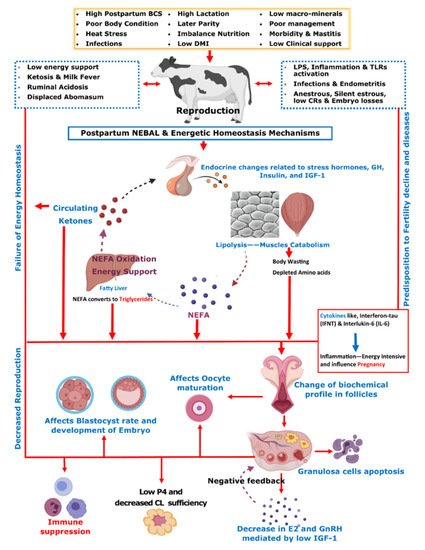
3. The Impact of Ketosis on Reproductive Efficiency of Dairy Cattle
3.1. Ketosis and Ovarian Dynamics
3.2. Ketosis Association with Oocyte Maturation and Implantation
4. The Impact of Hypocalcemia (Milk Fever) on Reproductive Efficiency of Dairy Cattle
5. Effect of High-Protein Diet on Reproductive Performance
References
- Dobson, H.; Smith, R.; Royal, M.; Knight, C.; Sheldon, I. The high-producing dairy cow and its reproductive performance. Reprod. Domest. Anim. 2007, 42 (Suppl. S2), 17–23.
- Lucy, M.C. Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? J. Dairy Sci. 2001, 84, 1277–1293.
- Pryce, J.E.; Royal, M.D.; Garnsworthy, P.C.; Mao, I.L. Fertility in the high-producing dairy cow. Livest. Prod. Sci. 2004, 86, 125–135.
- Lucy, M.C. Symposium review: Selection for fertility in the modern dairy cow—Current status and future direction for genetic selection. Dairy Sci. 2019, 102, 3706–3721.
- Thatcher, W.W.; Bilby, T.R.; Bartolome, J.A.; Silvestre, F.; Staples, C.R.; Santos, J.E.P. Strategies for improving fertility in the modern dairy cow. Theriogenology 2006, 65, 30–44.
- Stevenson, J.S.; Call, E.P. Reproductive Disorders in the Periparturient Dairy Cow. J. Dairy Sci. 1988, 71, 2572–2583.
- Fourichon, C.; Seegers, H.; Malher, X. Effect of disease on reproduction in the dairy cow: A meta-analysis. Theriogenology 2000, 53, 1729–1759.
- Mohtashamipour, F.; Dirandeh, E.; Ansari-pirsaraei, Z.; Colazo, M.G. Postpartum health disorders in lactating dairy cows and its associations with reproductive responses and pregnancy status after first timed-AI. Theriogenology 2020, 141, 98–104.
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986.
- Häggman, J.; Christensen, J.M.; Mäntysaari, E.A.; Juga, J. Genetic parameters for endocrine and traditional fertility traits, hyperketonemia and milk yield in dairy cattle. Animal 2019, 13, 248–255.
- Toledo-Alvarado, H.; Cecchinato, A.; Bittante, G. Fertility traits of Holstein, Brown Swiss, Simmental, and Alpine Grey cows are differently affected by herd productivity and milk yield of individual cows. J. Dairy Sci. 2017, 100, 8220–8231.
- Song, Y.; Wang, Z.; Zhao, C.; Bai, Y.; Xia, C.; Xu, C. Effect of negative energy balance on plasma metabolites, minerals, hormones, cytokines and ovarian follicular growth rate in Holstein dairy cows. J. Vet. Res. 2021, 65, 361–368.
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 5056–5066.
- Yoo, H.S. Infectious causes of reproductive disorders in cattle. J. Reprod. Dev. 2010, 56, S53–S60.
- Miqueo, E.; Chiarle, A.; Giuliodori, M.J.; Relling, A.E. Association between prepartum metabolic status and resumption of postpartum ovulation in dairy cows. Domest. Anim. Endocrinol. 2019, 69, 62–67.
- Roth, Z.; Meiden, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000, 120, 83–90.
- LeBlanc, S.J.; Lissemore, K.D.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 2006, 89, 1267–1279.
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2223–2236.
- Vergara, C.F.; Döpfer, D.; Cook, N.B.; Nordlund, K.V.; McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Risk factors for postpartum problems in dairy cows: Explanatory and predictive modeling. J. Dairy Sci. 2014, 97, 4127–4140.
- Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Gomes, G.C.; Greco, L.F.; Tao, S.; Thompson, I.M.; do Amaral, B.C.; Zenobi, M.G.; Martinez, N.; et al. Association of dry matter intake and energy balance prepartum and postpartum with health disorders postpartum: Part II. Ketosis and clinical mastitis. J. Dairy Sci. 2019, 102, 9151–9164.
- Hayirli, A.; Grummer, R.R.; Nordheim, E.V.; Crump, P.M. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J. Dairy Sci. 2002, 85, 3430–3443.
- Grummer, R.R.; Mashek, D.G.; Hayirli, A. Dry matter intake and energy balance in the transition period. Vet. Clin. N. Am.-Food Anim. Pract. 2004, 20, 447–470.
- Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Gomes, G.C.; Greco, L.F.; Tao, S.; Toledo, I.M.; do Amaral, B.C.; Zenobi, M.G.; Martinez, N.; et al. Investigating the Use of Dry Matter Intake and Energy Balance Prepartum as Predictors of Digestive Disorders Postpartum. Front. Vet. Sci. 2021, 8, 1016.
- Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Gomes, G.C.; Greco, L.F.; Tao, S.; Thompson, I.M.; do Amaral, B.C.; Zenobi, M.G.; Martinez, N.; et al. Association of dry matter intake and energy balance prepartum and postpartum with health disorders postpartum: Part I. Calving disorders and metritis. J. Dairy Sci. 2019, 102, 9138–9150.
- Hoedemaker, M.; Prange, D.; Gundelach, Y. Body condition change ante- and postpartum, health and reproductive performance in German Holstein Cows. Reprod. Domest. Anim. 2009, 44, 167–173.
- Chebel, R.C.; Mendonça, L.G.D.; Baruselli, P.S. Association between body condition score change during the dry period and postpartum health and performance. J. Dairy Sci. 2018, 101, 4595–4614.
- Zhang, F.; Li, D.; Wu, Q.; Sun, J.; Guan, W.; Hou, Y.; Zhu, Y.; Wang, J. Prepartum body conditions affect insulin signaling pathways in postpartum adipose tissues in transition dairy cows. J. Anim. Sci. Biotechnol. 2019, 10, 38.
- Roche, J.R.; Meier, S.; Heiser, A.; Mitchell, M.D.; Walker, C.G.; Crookenden, M.A.; Riboni, M.V.; Loor, J.J.; Kay, J.K. Effects of precalving body condition score and prepartum feeding level on production, reproduction, and health parameters in pasture-based transition dairy cows. J. Dairy Sci. 2015, 98, 7164–7182.
- Pires, J.A.A.; Delavaud, C.; Faulconnier, Y.; Pomiès, D.; Chilliard, Y. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows. J. Dairy Sci. 2013, 96, 6423–6439.
- Çolakoğlu, H.E.; Yazlık, M.O.; Pekcan, M.; Kaya, U.; Kaçar, C.; Vural, M.R.; Kurt, S.; Yildirim, M.M.; Bas, A.; Küplülü, Ş. Impact of prepartum body condition score loss on metabolic status during the transition period and subsequent fertility in Brown Swiss dairy cows. J. Vet. Res. 2019, 63, 375.
- Alharthi, A.S.; Coleman, D.N.; Alhidary, I.A.; Abdelrahman, M.M.; Trevisi, E.; Loor, J.J. Maternal body condition during late-pregnancy is associated with in utero development and neonatal growth of Holstein calves. J. Anim. Sci. Biotechnol. 2021, 12, 44.
- Reddy, N.M.; Potteti, H.R.; Vegiraju, S.; Chen, H.J.; Tamatam, C.M.; Reddy, S.P. PI3K-AKT Signaling via Nrf2 Protects against Hyperoxia-Induced Acute Lung Injury, but Promotes Inflammation Post-Injury Independent of Nrf2 in Mice. PLoS ONE 2015, 10, e0129676.
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional role of PPARs in ruminants: Potential targets for fine-tuning metabolism during growth and lactation. PPAR Res. 2013, 2013, 684159.
- Shinsyu, A.; Bamba, S.; Kurihara, M.; Matsumoto, H.; Sonoda, A.; Inatomi, O.; Andoh, A.; Takebayashi, K.; Kojima, M.; Iida, H.; et al. Inflammatory cytokines, appetite-regulating hormones, and energy metabolism in patients with gastrointestinal cancer. Oncol. Lett. 2020, 20, 1469–1479.
- Celeska, I.; Janevski, A.; Dzadzovski, I.; Ulchar, I.; Kirovski, D. The dynamics of biochemical parameters in blood of clinically healthy Holstein cows from day 5 before to day 60 after calving. Maced. Vet. Rev. 2015, 38, 189–193.
- Gross, J.J.; Bruckmaier, R.M. Review: Metabolic challenges in lactating dairy cows and their assessment via established and novel indicators in milk. Animal 2019, 13, s75–s81.
- Bach, À. Effects of nutrition and genetics on fertility in dairy cows. Reprod. Fertil. Dev. 2019, 31, 40–54.
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529.
- Sundrum, A. Metabolic Disorders in the Transition Period Indicate that the Dairy Cows’ Ability to Adapt is Overstressed. Animals 2015, 5, 978–1020.
- Abdelli, A.; Raboisson, D.; Kaidi, R.; Ibrahim, B.; Kalem, A.; Iguer-Ouada, M. Elevated non-esterified fatty acid and β-hydroxybutyrate in transition dairy cows and their association with reproductive performance and disorders: A meta-analysis. Theriogenology 2017, 93, 99–104.
- Cardoso, F.C.; Kalscheur, K.F.; Drackley, J.K. Symposium review: Nutrition strategies for improved health, production, and fertility during the transition period. J. Dairy Sci. 2020, 103, 5684–5693.
- Galligan, D. Economic assessment of animal health performance. Vet. Clin. N. Am.-Food Anim. Pract. 2006, 22, 207–227.
- Ceciliani, F.; Lecchi, C.; Urh, C.; Sauerwein, H. Proteomics and metabolomics characterizing the pathophysiology of adaptive reactions to the metabolic challenges during the transition from late pregnancy to early lactation in dairy cows. J. Proteomics 2018, 178, 92–106.
- Ribeiro, E.S.; Gomes, G.; Greco, L.F.; Cerri, R.L.A.; Vieira-Neto, A.; Monteiro, P.L.J.; Lima, F.S.; Bisinotto, R.S.; Thatcher, W.W.; Santos, J.E.P. Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows. J. Dairy Sci. 2016, 99, 2201–2220.
- Carvalho, M.R.; Peñagaricano, F.; Santos, J.E.P.; DeVries, T.J.; McBride, B.W.; Ribeiro, E.S. Long-term effects of postpartum clinical disease on milk production, reproduction, and culling of dairy cows. J. Dairy Sci. 2019, 102, 11701–11717.
- Garzón-Audor, A.; Oliver-Espinosa, O. Incidence and risk factors for ketosis in grazing dairy cattle in the Cundi-Boyacencian Andean plateau, Colombia. Trop. Anim. Health Prod. 2019, 51, 1481–1487.
- Brunner, N.; Groeger, S.; Canelas Raposo, J.; Bruckmaier, R.M.; Gross, J.J. Prevalence of subclinical ketosis and production diseases in dairy cows in Central and South America, Africa, Asia, Australia, New Zealand, and Eastern Europe. Transl. Anim. Sci. 2019, 3, 84–92.
- Sepúlveda-Varas, P.; Weary, D.M.; Noro, M.; Von Keyserlingk, M.A.G. Transition Diseases in Grazing Dairy Cows Are Related to Serum Cholesterol and Other Analytes. PLoS ONE 2015, 10, e0122317.
- Lucy, M.C. Regulation of Ovarian Follicular Growth by Somatotropin and Insulin-Like Growth Factors in Cattle. J. Dairy Sci. 2000, 83, 1635–1647.
- Bauman, D.E.; Vernon, R.G. Effects of Exogenous Bovine Somatotropin on Lactation. Annu. Rev. Nutr. 1993, 13, 437–461.
- Ghanem, M.E.; Tezuka, E.; Sasaki, K.; Takahashi, M.; Yamagishi, N.; Izaike, Y.; Osawa, T. Correlation of blood metabolite concentrations and body condition scores with persistent postpartum uterine bacterial infection in dairy cows. J. Reprod. Dev. 2016, 62, 457–463.
- Beam, S.W.; Butler, W.R. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J. Reprod. Fertil. Suppl. 1999, 54, 411–424.
- Rhoads, R.P.; Kim, J.W.; Leury, B.J.; Baumgard, L.H.; Segoale, N.; Frank, S.J.; Bauman, D.E.; Boisclair, Y.R. Insulin Increases the Abundance of the Growth Hormone Receptor in Liver and Adipose Tissue of Periparturient Dairy Cows. J. Nutr. 2004, 134, 1020–1027.
- Jorritsma, R.; Wensing, T.; Kruip, T.A.M.; Vos, P.L.A.M.; Noordhuizen, J.P.T.M. Metabolic changes in early lactation and impaired reproductive performance in dairy cows. Vet. Res. 2003, 34, 11–26.
- Butler, W.R. Nutritional effects on resumption of ovarian cyclicity and conception rate in postpartum dairy cows. BSAP Occas. Publ. 2001, 26, 133–145.
- Castro, N.; Kawashima, C.; van Dorland, H.A.; Morel, I.; Miyamoto, A.; Bruckmaier, R.M. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J. Dairy Sci. 2012, 95, 5804–5812.
- Villa-Godoy, A.; Hughes, T.L.; Emery, R.S.; Chapin, L.T.; Fogwell, R.L. Association Between Energy Balance and Luteal Function in Lactating Dairy Cows. J. Dairy Sci. 1988, 71, 1063–1072.
- Spicer, L.J.; Tucker, W.B.; Adams, G.D. Insulin-Like Growth Factor-I in Dairy Cows: Relationships Among Energy Balance, Body Condition, Ovarian Activity, and Estrous Behavior. J. Dairy Sci. 1990, 73, 929–937.
- Leroy, J.L.M.R.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; de Kruif, A.; Genicot, G.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495.
- Leroy, J.L.M.R.; Vanholder, T.; Van Knegsel, A.T.M.; Garcia-Ispierto, I.; Bols, P.E.J. Nutrient Prioritization in Dairy Cows Early Postpartum: Mismatch Between Metabolism and Fertility? Reprod. Domest. Anim. 2008, 43, 96–103.
- Seifi, H.A.; Gorji-Dooz, M.; Mohri, M.; Dalir-Naghadeh, B.; Farzaneh, N. Variations of energy-related biochemical metabolites during transition period in dairy cows. Comp. Clin. Path. 2007, 253–258.
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603.
- Wathes, D.C.; Fenwick, M.; Cheng, Z.; Bourne, N.; Llewellyn, S.; Morris, D.G.; Kenny, D.; Murphy, J.; Fitzpatrick, R. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow. Theriogenology 2007, 68, S232–S241.
- Velázquez, M.M.L.; Peralta, M.B.; Angeli, E.; Stassi, A.F.; Gareis, N.C.; Durante, L.; Cainelli, S.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim. Reprod. Sci. 2019, 206, 1–10.
- Zhang, Y.; Li, X.; Zhang, H.; Zhao, Z.; Peng, Z.; Wang, Z.; Liu, G.; Li, X. Non-Esterified Fatty Acids Over-Activate the TLR2/4-NF-Κb Signaling Pathway to Increase Inflammatory Cytokine Synthesis in Neutrophils from Ketotic Cows. Cell. Physiol. Biochem. 2018, 48, 827–837.
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650.
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Ramesha, K.P.; Sejian, V.; Rajendran, D.; Varghese, M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World 2017, 10, 1367.
- Douglas, G.N.; Overton, T.R.; Bateman, H.G.; Dann, H.M.; Drackley, J.K. Prepartal Plane of Nutrition, Regardless of Dietary Energy Source, Affects Periparturient Metabolism and Dry Matter Intake in Holstein Cows. J. Dairy Sci. 2006, 89, 2141–2157.
- Sammad, A.; Luo, H.; Qiu, W.; Galindez, J.M.; Wang, Y.; Guo, G.; Huang, X.; Wang, Y. Automated monitoring of seasonal and diurnal variation of rumination behaviour: Insights into thermotolerance management of Holstein cows. Biosyst. Eng. 2021.
- Cardoso, F.C.; LeBlanc, S.J.; Murphy, M.R.; Drackley, J.K. Prepartum nutritional strategy affects reproductive performance in dairy cows. J. Dairy Sci. 2013, 96, 5859–5871.
- Janovick, N.A.; Drackley, J.K. Prepartum dietary management of energy intake affects postpartum intake and lactation performance by primiparous and multiparous Holstein cows1. J. Dairy Sci. 2010, 93, 3086–3102.
- Drackley, J.K.; Cicela, T.M.; LaCount, D.W. Responses of primiparous and multiparous holstein cows to additional energy from fat or concentrate during summer. J. Dairy Sci. 2003, 86, 1306–1314.
- Ji, P.; Osorio, J.S.; Drackley, J.K.; Loor, J.J. Overfeeding a moderate energy diet prepartum does not impair bovine subcutaneous adipose tissue insulin signal transduction and induces marked changes in peripartal gene network expression. J. Dairy Sci. 2012, 95, 4333–4351.
- Graugnard, D.E.; Bionaz, M.; Trevisi, E.; Moyes, K.M.; Salak-Johnson, J.L.; Wallace, R.L.; Drackley, J.K.; Bertoni, G.; Loor, J.J. Blood immunometabolic indices and polymorphonuclear neutrophil function in peripartum dairy cows are altered by level of dietary energy prepartum. J. Dairy Sci. 2012, 95, 1749–1758.
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71.
- Nazeer, M.; Kumar, S.; Jaiswal, M.; Mishra, A.; Upmanyu, G.; Kumar, P.; Kumar, S.A. Prevalence and Clinical Manifestations of Ketosis in Cows in and Around Bikaner. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1554–1560.
- Galster, A.D.; Clutter, W.E.; Cryer, P.E.; Collins, J.A.; Bier, D.M. Epinephrine plasma thresholds for lipolytic effects in man: Measurements of fatty acid transport with palmitic acid. J. Clin. Investig. 1981, 67, 1729–1738.
- Melendez, P.; Marin, M.P.; Robles, J.; Rios, C.; Duchens, M.; Archbald, L. Relationship between serum nonesterified fatty acids at calving and the incidence of periparturient diseases in Holstein dairy cows. Theriogenology 2009, 72, 826–833.
- Duffield, T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 231–253.
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. N. Am.-Food Anim. Pract. 2004, 20, 651–674.
- Staufenbiel, R.; Arndt, G.; Schröder, U.; Gelfert, C.C. Body condition and metabolic stability as the basis for high milk yield and undisturbed fertility in dairy cows—A contribution for deduction of reference values. Dtsch. Tierarztl. Wochenschr. 2004, 111, 214–220.
- Mostert, P.F.; Bokkers, E.A.M.; Van Middelaar, C.E.; Hogeveen, H.; De Boer, I.J.M. Estimating the economic impact of subclinical ketosis in dairy cattle using a dynamic stochastic simulation model. Animal 2018, 12, 145–154.
- Diskin, M.G.; Mackey, D.R.; Roche, J.F.; Sreenan, J.M. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 2003, 78, 345–370.
- Pushpakumara, P.G.A.; Gardner, N.H.; Reynolds, C.K.; Beever, D.E.; Wathes, D.C. Relationships between transition period diet, metabolic parameters and fertility in lactating dairy cows. Theriogenology 2003, 60, 1165–1185.
- Chapinal, N.; LeBlanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.P.; Overton, M.W.; Duffield, T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682.
- Shin, E.K.; Jeong, J.K.; Choi, I.S.; Kang, H.G.; Hur, T.Y.; Jung, Y.H.; Kim, I.H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology 2015, 84, 252–260.
- Lüttgenau, J.; Purschke, S.; Tsousis, G.; Bruckmaier, R.M.; Bollwein, H. Body condition loss and increased serum levels of nonesterified fatty acids enhance progesterone levels at estrus and reduce estrous activity and insemination rates in postpartum dairy cows. Theriogenology 2016, 85, 656–663.
- Rutherford, A.J.; Oikonomou, G.; Smith, R.F. The effect of subclinical ketosis on activity at estrus and reproductive performance in dairy cattle. J. Dairy Sci. 2016, 99, 4808–4815.
- Aardema, H.; van Tol, H.T.A.; Vos, P.L.A.M. An overview on how cumulus cells interact with the oocyte in a condition with elevated NEFA levels in dairy cows. Anim. Reprod. Sci. 2019, 207, 131–137.
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138.
- Piechotta, M.; Mysegades, W.; Ligges, U.; Lilienthal, J.; Hoeflich, A.; Miyamoto, A.; Bollwein, H. Antepartal insulin-like growth factor 1 and insulin-like growth factor binding protein 2 concentrations are indicative of ketosis in dairy cows. J. Dairy Sci. 2015, 98, 3100–3109.
- Sarentonglaga, B.; Ogata, K.; Taguchi, Y.; Kato, Y.; Nagao, Y. The developmental potential of oocytes is impaired in cattle with liver abnormalities. J. Reprod. Dev. 2013, 59, 168–173.
- Valckx, S.D.M.; Arias-Alvarez, M.; De Pauw, I.; Fievez, V.; Vlaeminck, B.; Fransen, E.; Bols, P.E.J.; Leroy, J.L.M.R. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: A descriptive cross-sectional study. Reprod. Biol. Endocrinol. 2014, 12, 13.
- Aardema, H.; Gadella, B.M.; van de Lest, C.H.A.; Brouwers, J.F.H.M.; Stout, T.A.E.; Roelen, B.A.J.; Vos, P.L.A.M. Free fatty acid levels in fluid of dominant follicles at the preferred insemination time in dairy cows are not affected by early postpartum fatty acid stress. J. Dairy Sci. 2015, 98, 2322–2336.
- Cheng, Y.; Liu, S.; Lin, R.; Wang, J.; Peng, T.; Zhang, Q.; Cheng, H. Plasma and amniotic fluid PPARγ is involved in the lipid metabolism of maternal–fetal interface cells. J. Matern. Neonatal Med. 2018, 31, 2656–2664.
- Furukawa, E.; Chen, Z.; Ueshiba, H.; Wu, Y.; Chiba, H.; Yanagawa, Y.; Katagiri, S.; Nagano, M.; Hui, S.P. Postpartum cows showed high oocyte triacylglycerols concurrently with high plasma free fatty acids. Theriogenology 2021, 176, 174–182.
- Campanile, G.; Baruselli, P.S.; Limone, A.; D’Occhio, M.J. Local action of cytokines and immune cells in communication between the conceptus and uterus during the critical period of early embryo development, attachment and implantation – Implications for embryo survival in cattle: A review. Theriogenology 2021, 167, 1–12.
- van Mourik, M.S.M.; Macklon, N.S.; Heijnen, C.J. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009, 85, 4–19.
- Lacasse, P.; Vanacker, N.; Ollier, S.; Ster, C. Innovative dairy cow management to improve resistance to metabolic and infectious diseases during the transition period. Res. Vet. Sci. 2018, 116, 40–46.
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Periparturient immunosuppression and strategies to improve dairy cow health during the periparturient period. Res. Vet. Sci. 2016, 108, 8–17.
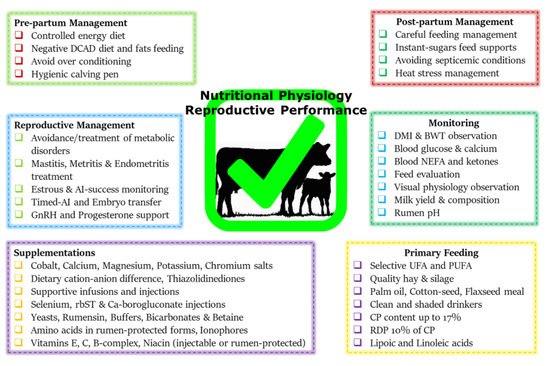
- Drackley, J.K.; Cardoso, F.C. Prepartum and postpartum nutritional management to optimize fertility in high-yielding dairy cows in confined TMR systems. Animal 2014, 8, 5–14.
- Garnsworthy, P.C.; Sinclair, K.D.; Webb, R. Integration of physiological mechanisms that influence fertility in dairy cows. Animal 2008, 2, 1144–1152.
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57.
- Zhang, F.; Nan, X.; Wang, H.; Guo, Y.; Xiong, B. Research on the applications of calcium propionate in dairy cows: A review. Animals 2020, 10, 1336.
- Hernández-Castellano, L.E.; Hernandez, L.L.; Bruckmaier, R.M. Review: Endocrine pathways to regulate calcium homeostasis around parturition and the prevention of hypocalcemia in periparturient dairy cows. Animal 2020, 14, 330–338.
- Özçelik, R.; Bruckmaier, R.M.; Hernández-Castellano, L.E. Prepartum daylight exposure increases serum calcium concentrations in dairy cows at the onset of lactation. J. Anim. Sci. 2017, 95, 4440–4447.
- Wysolmerski, J.J. Osteocytic osteolysis: Time for a second look? Bonekey Rep. 2012, 1, 229.
- Megahed, A.A.; Hiew, M.W.H.; El Badawy, S.A.; Constable, P.D. Plasma calcium concentrations are decreased at least 9 hours before parturition in multiparous Holstein-Friesian cattle in a herd fed an acidogenic diet during late gestation. J. Dairy Sci. 2018, 101, 1365–1378.
- Umaña Sedó, S.; Rosa, D.; Mattioli, G.; Luzbel de la Sota, R.; Giuliodori, M.J. Associations of subclinical hypocalcemia with fertility in a herd of grazing dairy cows. J. Dairy Sci. 2018, 101, 10469–10477.
- Saborío-Montero, A.; Vargas-Leitón, B.; Romero-Zúñiga, J.J.; Sánchez, J.M. Risk factors associated with milk fever occurrence in grazing dairy cattle. J. Dairy Sci. 2017, 100, 9715–9722.
- Chiwome, B.; Kandiwa, E.; Mushonga, B.; Sajeni, S.; Habarugira, G. A study of the incidence of milk fever in Jersey and Holstein cows at a dairy farm in Beatrice, Zimbabwe. J. S. Afr. Vet. Assoc. 2017, 88, 6.
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69.
- Venjakob, P.L.; Borchardt, S.; Heuwieser, W. Hypocalcemia—Cow-level prevalence and preventive strategies in German dairy herds. J. Dairy Sci. 2017, 100, 9258–9266.
- Lean, I.J.; DeGaris, P.J.; McNeil, D.M.; Block, E. Hypocalcemia in dairy cows: Meta-analysis and dietary cation anion difference theory revisited. J. Dairy Sci. 2006, 89, 669–684.
- Caixeta, L.S.; Ospina, P.A.; Capel, M.B.; Nydam, D.V. Association between subclinical hypocalcemia in the first 3 days of lactation and reproductive performance of dairy cows. Theriogenology 2017, 94, 1–7.
- Correa, M.T.; Erb, H.; Scarlett, J. Path Analysis for Seven Postpartum Disorders of Holstein Cows. J. Dairy Sci. 1993, 76, 1305–1312.
- Markusfeld, O. Periparturient Traits in Seven High Dairy Herds. Incidence Rates, Association with Parity, and Interrelationships Among Traits. J. Dairy Sci. 1987, 70, 158–166.
- Jeong, J.K.; Kang, H.G.; Kim, I.H. Associations between serum calcium concentration and postpartum health and reproductive performance in dairy cows. Anim. Reprod. Sci. 2018, 196, 184–192.
- Thys-Jacobs, S.; Donovan, D.; Papadopoulos, A.; Sarrel, P.; Bilezikian, J.P. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 1999, 64, 430–435.
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of subclinical hypocalcemia in dairy herds. Vet. J. 2011, 188, 122–124.
- Neves, R.C.; Leno, B.M.; Stokol, T.; Overton, T.R.; McArt, J.A.A. Risk factors associated with postpartum subclinical hypocalcemia in dairy cows. J. Dairy Sci. 2017, 100, 3796–3804.
- Chapinal, N.; Carson, M.; Duffield, T.F.; Capel, M.; Godden, S.; Overton, M.; Santos, J.E.P.; LeBlanc, S.J. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 2011, 94, 4897–4903.
- Rodríguez, E.M.; Arís, A.; Bach, A. Associations between subclinical hypocalcemia and postparturient diseases in dairy cows. J. Dairy Sci. 2017, 100, 7427–7434.
- Oetzel, G.R. Parturient paresis and hypocalcemia in ruminant livestock. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 351–364.
- Leno, B.M.; Ryan, C.M.; Stokol, T.; Kirk, D.; Zanzalari, K.P.; Chapman, J.D.; Overton, T.R. Effects of prepartum dietary cation-anion difference on aspects of peripartum mineral and energy metabolism and performance of multiparous Holstein cows. J. Dairy Sci. 2017, 100, 4604–4622.
- Weich, W.; Block, E.; Litherland, N.B. Extended negative dietary cation-anion difference feeding does not negatively affect postpartum performance of multiparous dairy cows. J. Dairy Sci. 2013, 96, 5780–5792.
- Lopera, C.; Zimpel, R.; Vieira-Neto, A.; Lopes, F.R.; Ortiz, W.; Poindexter, M.; Faria, B.N.; Gambarini, M.L.; Block, E.; Nelson, C.D.; et al. Effects of level of dietary cation-anion difference and duration of prepartum feeding on performance and metabolism of dairy cows. J. Dairy Sci. 2018, 101, 7907–7929.
- Ryan, K.T.; Guadagnin, A.R.; Glosson, K.M.; Bascom, S.S.; Rowson, A.D.; Steelman, A.J.; Cardoso, F.C. Increased dietary calcium inclusion in fully acidified prepartum diets improved postpartum uterine health and fertility when fed to Holstein cows. Theriogenology 2020, 142, 338–347.
- Thilsing-Hansen, T.; Jørgensen, R.J.; Enemark, J.M.D.; Larsen, T. The effect of zeolite a supplementation in the dry period on periparturient calcium, phosphorus, and magnesium homeostasis. J. Dairy Sci. 2002, 85, 1855–1862.
- Kronqvist, C.; Emanuelson, U.; Spörndly, R.; Holtenius, K. Effects of prepartum dietary calcium level on calcium and magnesium metabolism in periparturient dairy cows. J. Dairy Sci. 2011, 94, 1365–1373.
- Kronqvist, C.; Emanuelson, U.; Tråvén, M.; Spãrndly, R.; Holtenius, K. Relationship between incidence of milk fever and feeding of minerals during the last 3 weeks of gestation. Animal 2012, 6, 1316–1321.
- Wächter, S.; Cohrs, I.; Golbeck, L.; Wilkens, M.R.; Grünberg, W. Effects of restricted dietary phosphorus supply to dry cows on periparturient calcium status. J. Dairy Sci. 2022, 105, 748–760.
- Martín-Tereso, J.; Martens, H.; Deiner, C.; Van Laar, H.; Den Hartog, L.A.; Verstegen, M.W.A. Pre-calving feeding of rumen-protected rice bran to multiparous dairy cows improves recovery of calcaemia after calving. J. Dairy Res. 2016, 83, 281–288.
- Khachlouf, K.; Hamed, H.; Gdoura, R.; Gargouri, A. Effects of dietary Zeolite supplementation on milk yield and composition and blood minerals status in lactating dairy cows. J. Appl. Anim. Res. 2019, 47, 54–62.
- Laporta, J.; Moore, S.A.E.; Peters, M.W.; Peters, T.L.; Hernandez, L.L. Short communication: Circulating serotonin (5-HT) concentrations on day 1 of lactation as a potential predictor of transition-related disorders. J. Dairy Sci. 2013, 96, 281–288.
- Slater, C.J.; Endres, E.L.; Weaver, S.R.; Cheng, A.A.; Lauber, M.R.; Endres, S.F.; Olstad, E.; DeBruin, A.; Crump, P.M.; Block, E.; et al. Interaction of 5-hydroxy-L-tryptophan and negative dietary cation-anion difference on calcium homeostasis in multiparous peripartum dairy cows. J. Dairy Sci. 2018, 101, 5486–5501.
- Wang, F.; Shi, H.; Wang, S.; Wang, Y.; Cao, Z.; Li, S. Amino Acid Metabolism in Dairy Cows and their Regulation in Milk Synthesis. Curr. Drug Metab. 2019, 20, 36–45.
- Butler, W.R. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 2000, 60–61, 449–457.
- Tamminga, S. The effect of the supply of rumen degradable protein and metabolisable protein on negative energy balance and fertility in dairy cows. Anim. Reprod. Sci. 2006, 96, 227–239.
- Campanile, G.; Di Palo, R.; Infascelli, F.; Gasparrini, B.; Neglia, G.; Zicarelli, F.; D’Occhio, M.J. Influence of rumen protein degradability on productive and reproductive performance in buffalo cows. Reprod. Nutr. Dev. 2003, 43, 557–566.
- Raboisson, D.; Albaaj, A.; Nonne, G.; Foucras, G. High urea and pregnancy or conception in dairy cows: A meta-analysis to define the appropriate urea threshold. J. Dairy Sci. 2017, 100, 7581–7587.
- Kananub, S.; Pechkerd, P.; VanLeeuwen, J.; Stryhn, H.; Arunvipas, P. Evaluation of influence of milk urea nitrogen on reproductive performance in smallholder dairy farms. Aust. Vet. J. 2020, 98, 375–379.
- Mohanty, P.; Ghanim, H.; Hamouda, W.; Aljada, A.; Garg, R.; Dandona, P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am. J. Clin. Nutr. 2002, 75, 767–772.
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49.
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian-Australas. J. Anim. Sci. 2018, 31, 47–53.
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337.
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: Consequences and opportunities. Animals 2020, 10, 793.
- Gao, H. Amino acids in reproductive nutrition and health. Adv. Exp. Med. Biol. 2020, 1265, 111–131.
- Elango, R.; Ball, R.O. Protein and Amino Acid Requirements during Pregnancy. Adv. Nutr. 2016, 7, 839S–844S.
- Ennis, M.; Lim, K.; Ball, R.; Pencharz, P.; Courtney-Martin, G.; Elango, R. Dietary Phenylalanine and Tyrosine Requirements in Healthy Human Pregnancy. Curr. Dev. Nutr. 2020, 4.
- Di Costanzo, A.; Spain, J.N.; Spiers, D.E. Supplementation of Nicotinic Acid for Lactating Holstein Cows Under Heat Stress Conditions. J. Dairy Sci. 1997, 80, 1200–1206.
- Hansen, P.J. Hidden factors affecting fertility. Adv. Dairy Technol. Proc. West. Can. Dairy Semin. 2007, 19, 339–349.
- Kaufman, J.D.; Pohler, K.G.; Mulliniks, J.T.; Ríus, A.G. Lowering rumen-degradable and rumen-undegradable protein improved amino acid metabolism and energy utilization in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2018, 101, 386–395.
- Havekes, C.D.; Duffield, T.F.; Carpenter, A.J.; DeVries, T.J. Effects of wheat straw chop length in high-straw dry cow diets on intake, health, and performance of dairy cows across the transition period. J. Dairy Sci. 2020, 103, 254–271.
- Acosta, D.A.V.; Rivelli, M.I.; Skenandore, C.; Zhou, Z.; Keisler, D.H.; Luchini, D.; Corrêa, M.N.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on steroidogenic potential of the first postpartum dominant follicle and expression of immune mediators in Holstein cows. Theriogenology 2017, 96, 1–9.
- Skenandore, C.S.; Velasco Acosta, D.A.; Zhou, Z.; Rivelli, M.I.; Corrêa, M.N.; Luchini, D.N.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on vaginal discharge and uterine cytology of Holstein cows. Int. J. Vet. Sci. Med. 2017, 5, 1–7.
- Stella, S.L.; Velasco-Acosta, D.A.; Skenandore, C.; Zhou, Z.; Steelman, A.; Luchini, D.; Cardoso, F.C. Improved uterine immune mediators in Holstein cows supplemented with rumen-protected methionine and discovery of neutrophil extracellular traps (NET). Theriogenology 2018, 114, 116–125.
- Acosta, D.A.V.; Denicol, A.C.; Tribulo, P.; Rivelli, M.I.; Skenandore, C.; Zhou, Z.; Luchini, D.; Corrêa, M.N.; Hansen, P.J.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on the preimplantation embryo in Holstein cows. Theriogenology 2016, 85, 1669–1679.
- Palmquist, D.L.; Jenkins, T.C. Fat in Lactation Rations: Review. J. Dairy Sci. 1980, 63, 1–14.
- Bicalho, M.L.S.; Lima, F.S.; Ganda, E.K.; Foditsch, C.; Meira, E.B.S.; Machado, V.S.; Teixeira, A.G.V.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Effect of trace mineral supplementation on selected minerals, energy metabolites, oxidative stress, and immune parameters and its association with uterine diseases in dairy cattle. J. Dairy Sci. 2014, 97, 4281–4295.
- Mammi, L.M.E.; Guadagnini, M.; Mechor, G.; Cainzos, J.M.; Fusaro, I.; Palmonari, A.; Formigoni, A. The Use of Monensin for Ketosis Prevention in Dairy Cows during the Transition Period: A Systematic Review. Animals 2021, 11, 1988.
- Silva, P.R.B.; Machado, K.S.; Da Silva, D.N.L.; Moraes, J.G.N.; Keisler, D.H.; Chebel, R.C. Effects of recombinant bovine somatotropin during the periparturient period on innate and adaptive immune responses, systemic inflammation, and metabolism of dairy cows. J. Dairy Sci. 2015, 98, 4449–4464.
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655.
- Hansen, P.J.; Aréchiga, C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999, 77 (Suppl. 2), 36–50.
- Cartmill, J.A.; El-Zarkouny, S.Z.; Hensley, B.A.; Rozell, T.G.; Smith, J.F.; Stevenson, J.S. An Alternative AI Breeding Protocol for Dairy Cows Exposed to Elevated Ambient Temperatures before or after Calving or Both. J. Dairy Sci. 2001, 84, 799–806.
- Stewart, B.M.; Block, J.; Morelli, P.; Navarette, A.E.; Amstalden, M.; Bonilla, L.; Hansen, P.J.; Bilby, T.R. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J. Dairy Sci. 2011, 94, 3437–3445.
- López-Gatius, F.; Santolaria, P.; Martino, A.; Delétang, F.; De Rensis, F. The effects of GnRH treatment at the time of AI and 12 days later on reproductive performance of high producing dairy cows during the warm season in northeastern Spain. Theriogenology 2006, 65, 820–830.
- Friedman, E.; Voet, H.; Reznikov, D.; Wolfenson, D.; Roth, Z. Hormonal treatment before and after artificial insemination differentially improves fertility in subpopulations of dairy cows during the summer and autumn. J. Dairy Sci. 2014, 97, 7465–7475.
