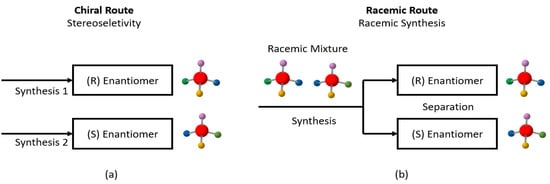
Enantiomers share the same chemical formula but have different chemical structures, i.e., type of isomers. Enantiomers are present in several drugs, perfumes, food, and are a fundamental part of biomolecules. This subject is highly important for pharmaceutical companies. Enantiomeric drugs present different actuation in the human body; depending on the compound, one might combat the symptom, whereas its pair might cause damage. The separation of pairs of enantiomers requires a chiral environment that provokes a structural imbalance that conventional methods cannot provide. Enantioresolution is one of the most promissory studies that benefit several areas, such as pharmaceutical, biotechnology, food industry, and fine chemistry. Its resolution is of great importance, therefore, its main mechanisms of resolution will be explained herein.
- enantiomer
- chirality
- nomenclature
- market
- enantioresolution
1. Introduction
| Isomerism | Chemical Formula | Structural Formula | |||
|---|---|---|---|---|---|
| Structural | Function | C3H6O |  |
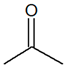 |
|
| Propanal | Propanone | ||||
| Chain | C4H10 |  |
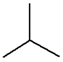 |
||
| n-Butane | Isobutane | ||||
| Position | C5H10O | 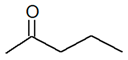 |
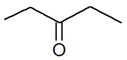 |
||
| 2-Pentanone | 3-Pentanone | ||||
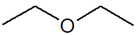
| Metamerism | C4H10O | 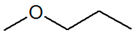 |
 |
||
| 1-Methoxypropane | Ethoxyethane | ||||
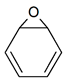
| Tautomerism | C6H6O | 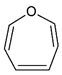 |
 |
 |
|
| Oxepin | Benzene oxide | ||||
| Stereo | Diastereomer (Geometric) |
C2H2Cl2 | 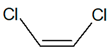 |
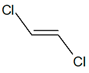 |
|
| cis-1,2-dichloroethene | trans-1,2-dichloroethene | ||||
| Enantiomer (Optical) |
C3H6O3 | 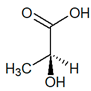 |
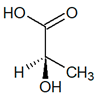 |
||
| (S)-Lactic acid | |||||
| Isomerism | Chemical Formula | Structural Formula | |||
| Structural | Function | C3H6O |  |
 |
|
| Propanal | Propanone | ||||
| Chain | C4H10 |  |
 |
||
| n-Butane | Isobutane | ||||
| Position | C5H10O |  |
 |
||
| 2-Pentanone | 3-Pentanone | ||||
| Metamerism | C4H10O |  |
 |
||
| 1-Methoxypropane | Ethoxyethane | ||||
| Tautomerism | C6H6O |  |
 |
 |
|
| Oxepin | Benzene oxide | ||||
| Stereo | Diastereomer (Geometric) |
C2H2Cl2 |  |
 |
|
| cis-1,2-dichloroethene | trans-1,2-dichloroethene | ||||
| Enantiomer (Optical) |
C3H6O3 |  |
 |
||
| (S)-Lactic acid | (R)-Lactic acid | ||||
| (R)-Lactic acid |
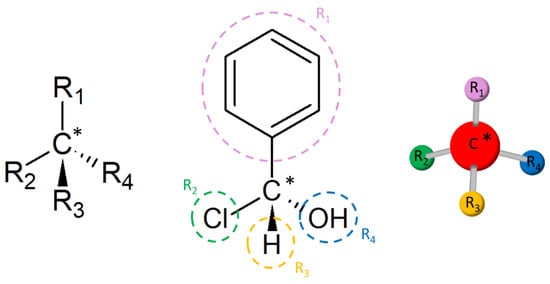
Optical activity is the phenomenon of shifting in the direction of the light plane when it passes through a compound. Light is an electromagnetic radiation that, when interacting with electrons in a molecule, slightly diverts its direction. Some molecules, though, do not present optical activity, i.e., for any light shift, either to the left or right, there is a molecule that shifts it in the opposite direction, nulling the optical activity. On the other hand, when light passes through pure there is a shift of the light; when light shifts to left, the enantiomer is called levorotatory (receives the prefix or (−)) and to the right it is called dextrorotatory (receives the prefix or (+)).
or (+)).where
is the specific rotation, superscript
designates the temperature (usually 20 °C), superscript
the light wavelength (usually at 589.6 nm of Na D-lines),
is the polarimeter length in dm,
is the enantiomer concentration in g·cm
, and
is the observed rotation in degrees in the polarimeter. It means that an
enantiomer has a negative specific rotation, a
enantiomer a positive one, and a racemate has a
of zero.
 (2)
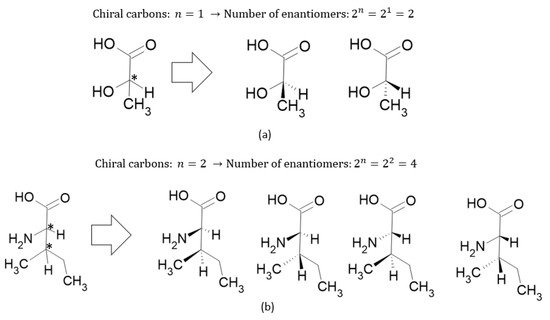
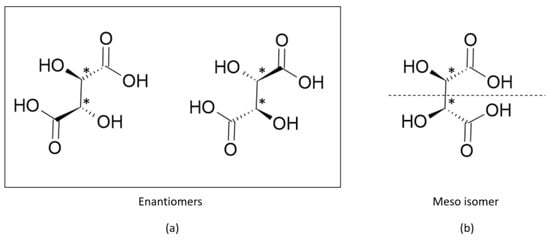
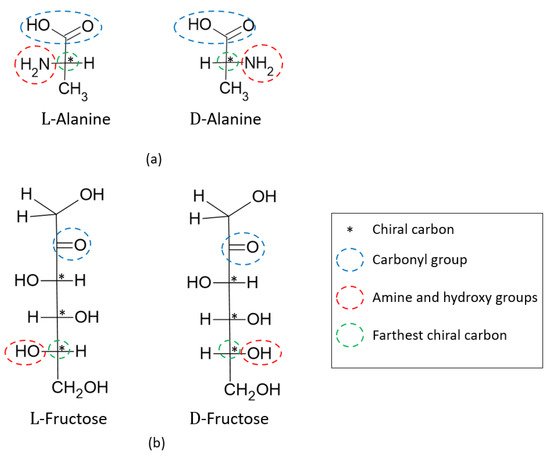
(2)where and are the number of enantiomers and chiral carbons, respectively, as exemplified in Figure 3. However, this correlation is not always true; molecules with at least two chiral carbons that present a plane of symmetry are not chiral molecules. One of its carbons shifts the light plan to left, whereas the other nulls its effect, an internal compensation. An example is illustrated in Figure 4, although the tartaric acid has two chiral carbons, it has only two enantiomers and one meso isomer, a diastereomer and a non-optically active stereoisomer (see Table 1).
2. Nomenclature



| Rule | Illustration | |
|---|---|---|
| 1st | Ranking of atoms in descending order of atomic number | 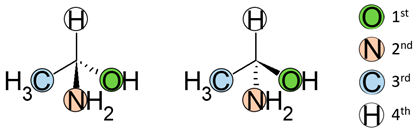 |
| 2nd | Pose the least atomic number atoms at the rear | 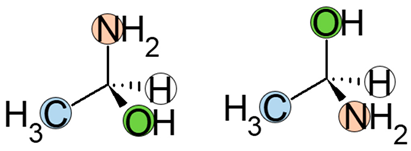 |
| 3rd | Draw a circle arrow from the first to the third position. |  |


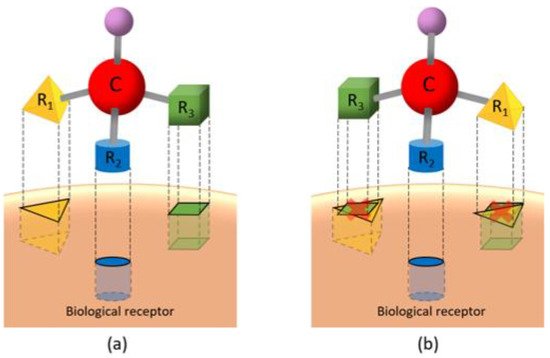
3. Enantiomers and the Human Body
| Compounds | (S) Enantiomer Actuation | (R) Enantiomer Actuation |
|---|---|---|
| Limonene | 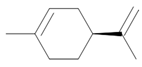 Lemon odor |
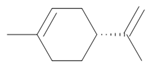 Orange odor |
| Carvone | 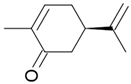 Caraway flavor |
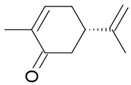 Spearmint flavor |
| Asparagine | 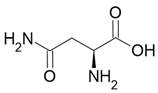 Bitter taste |
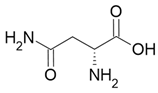 Sweet taste |
| Aspartame | 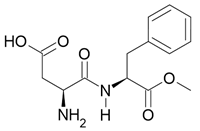 Sweet taste |
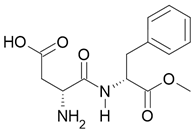 Bitter taste |
| Ethambutol | 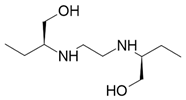 Tuberculostatic |
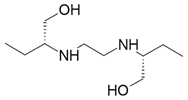 Causes blindness |
| Thalidomide | 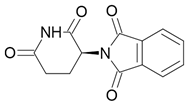 Teratogen |
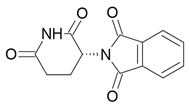 Sedative |
| Penicillamine | 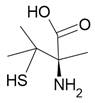 Antiarthritic |
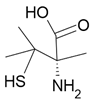 Mutagen |
| Ketamine | 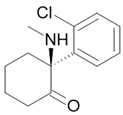 Anesthetic |
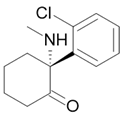 Hallucinogen |
| Dopa | 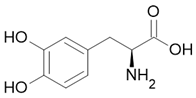 Anti-Parkinson |
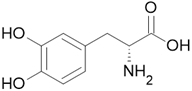 Serious side effects |
| Chloramphenicol | 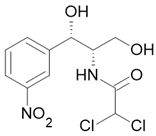 Inactive |
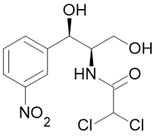 Antibacterial |
| Propranolol | 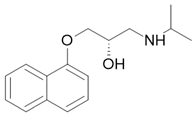 Antihypertensive, antiarrhythmic |
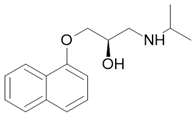 Contraceptive |
| Paclobutrazol | 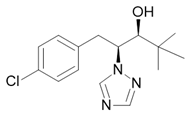 Plant growth regulator |
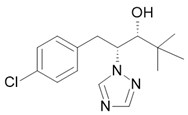 Fungicide |
4. Enantiomeric Drug Market
| Product Name | Chiral Active Ingredient | Indication | Revenue (in Millions of Dollars) |
|---|---|---|---|
| Revlimid | 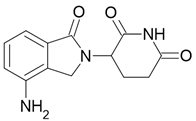 Lenalidomide |
Oncology | 8187 |
| Xarelto | 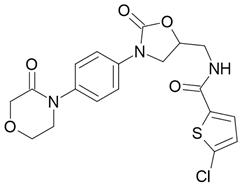 Rivaroxaban |
Cardiovascular Diseases | 6590 |
| Lyrica |  Pregabalin |
Neurological/Mental Disorders | 5317 |
| Imbruvica |  Ibrutinib |
Oncology | 4466 |
| Harvoni |  Ledipasvir  Sofosbuvir |
Infectious Diseases (HIV, Hepatitis, etc.) | 4370 |
| Symbicort Pulmicort |  Budesonide 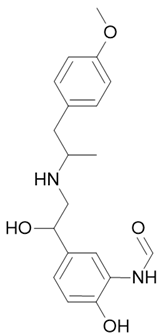 Formoterol |
Respiratory Disorders | 4360 |
| Januvia | 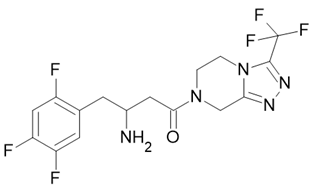 Sitagliptin |
Diabetes | 3737 |
| Epclusa | 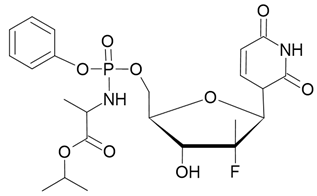 Sofosbuvir |
Infectious Diseases (HIV, Hepatitis, etc.) | 3510 |
| Triumeq | 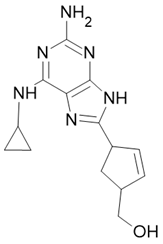 Abacavir  Dolutegravir  Lamivudine |
Infectious Diseases (HIV, Hepatitis, etc.) | 3470 |
| Latuda |  Lurasidone |
Neurological/Mental Disorders | 3350 |
| Truvada |  Emtricitabine |
Infectious Diseases (HIV, Hepatitis, etc.) | 3134 |
| Nexium |  Esomeprazol |
Gastrointestinal Disorders | 2795 |
| Invega Sustenna |  Paliperidone Palmitate |
Neurological/Mental Disorders | 2569 |
| Zytiga |  Abiraterone Acetate |
Oncology | 2505 |
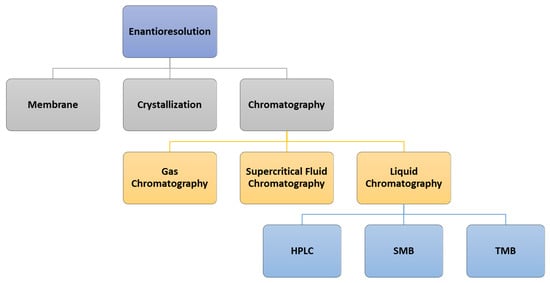
5. Enantioresolution
5.1. Crystallization
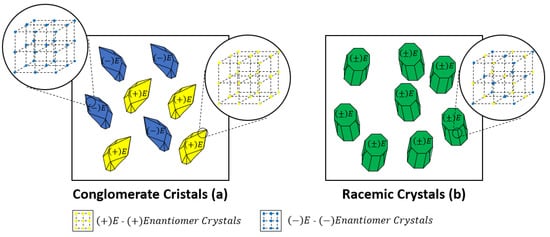
| Enantiomers | Ref. |
|---|---|
| Ketoprofen |
| Enantiomers | Membrane | Ref. | ||
|---|---|---|---|---|
| [36] | ||||
| Phenylalanine | Immobilized DNA membranes | [57] | 5-ethyl-5-methylhydantoin | [39 |
| Phenylalanine] | ||||
| DNA-immobilized chitosan membranes | [58] | Threonine | [42] | |
| Phenylalanine | Polyaniline | [60] | Aspartic acid and glutamic acid | [43] |
| Propranolol | [44] | |||
| N-methylamphetamine | [45] |
| Enantiomers | CSP | Ref. | |
|---|---|---|---|
| Ibuprofen | Kromasil CHI-TBB, Kromasil CHI-DMB, Chirobiotik T, Chiracel OBH and Chiralpal AD | [128] | |
| Dioxolane compounds | Chiralpak AD and Chiralcel OD | [129] | |
| Enantiomeric pharmaceuticals | Chiralpak AD | [130] | Agilent 6890 gas chromatograph |
5.3.3. LC—Liquid Chromatography
| Enantiomers | CSP | Eluent | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antifungal chiral drugs | Polysaccharide derivatives | Hexane-ethanol and hexane-2-propanol | [133] | |||||||
| Fungicide Enantiomers | Amylopectin Based Chiral | n-hexane and isopropanol | [144] | |||||||
| Threonine | ||||||||||
| Oxazepam, lorazepam, and temazepam | Derivatized cyclodextrin-bonded | Acetonitrile | [156] | Tryptophan, tyrosine, and phenylalanine | poly(γ-methyl-l-glutamate) membranes | Enantiomeric pharmaceuticalsHelium | Chiralcel OD, Chiralcel OJ and Chiralcel AD[61] | |||
| [ | 94 | ] | ||||||||
| [ | 131] | |||||||||
| 1,4-Dihydropyridines | Vancomycin | Methanol/acetic acid/TEA | [157] | Lactic acid and alanine | Alanina, prolina, serina, asparagine, glutamine, lisine, ornitinaPolypropylene hollow-fiber module liquid membrane | [62] | ||||
| Chirasil-l-Val capillary columns | Helium | [95] | ||||||||
| A set of 111 chiral compounds | Chirobiotic T, Chirobiotic TAG and Chirobiotic R | [132] | ||||||||
| Linezolid | Amylose based | Hexane, 2-propanol and trifluoro acetic acid | [158] | Naproxen | Obuprofen, fenoprofenPoly(4-vinylpyridine) /polypropylene membranes | and ketoprofen methyl esters |
Heptakis-(2,3-di-Omethyl- [63] |
6-O-t-butyldimethyl-silyl)-β-cyclodextrin | Hydrogen | [96] |
| Albendazole sulfoxide | Chiralpak AD and Chiralcel OD | [ | ||||||||
| Tangutorine | 133] | Chiralcel OD and Chiralpak AD | n-hexane/2-propanol | [159[46 | ||||||
| ] | Propranolol] | |||||||||
| Chiral derivatized polysulfone | [64] | Chiral epoxides | Cyclodextrin derivatives | Nitrogen | [97] | |||||
| Triadimefon and triadimenol | Chiralpak AD | [134]α-amino acids | ||||||||
| β-blockers | (R)-1-naphthylglycine and 3,5-dinitrobenzoic acid | Mandelic acid | [47] | |||||||
| Tryptophan, henylglycine and phenylalanine | Immobilized DNA membranes | [ | Modified Linear Dextrins65] | Hydrogen | [98] | Chiral microspheres based on poly(N-vinyl a-L-phenylalanine) | [ | |||
| Albendazole sulfoxide | Chiralpak AD | 1-phenylethanol48] | ||||||||
| [ | 135] | Methyl branched compounds | 2,3-Di-O-methoxymethyl-6-O-tert-butyldimethylsilyl-γ-cyclodextrin | Hydrogen | [99] | |||||
| n-hexane, 1,2-dichloroethane and methanol | [ | 160] | ||||||||
| Tolterodine tartarate | Chiralcel OD-H | n-hexane and isopropyl | [161] | Omeprazole and several related benzimidazoles(R,R)-TADDOL | [66] | Chiralpak AD | [136] | Benzo-(c)phenanthrene, 3,4-dehydroproline anhydride, and 2,6-dimethylglycoluril | [49] | |
| N-protected amino acid derivatives | Hydrocarbons, underivatized alcohols, ketones, and proteinogenic amino acid Adamantyl-carbamoyl-11- octadecylthioether-quinine/-quinidine |
[67] | derivatives | Permethylated-βcyclodextrin and resorcinarene with pendant L- or D-valine diamide groups |
||||||
| Triazole pesticides | Hydrogen | Chiralpak AD[100] | 2-(2-oxopyrrolidin-1-yl)butanamide | |||||||
| [ | 137] | [50] | ||||||||
| Tryptophan | Chitosan/-cyclodextrin composite membranes | [68] | β-Blockers | DB-5 and DB-17 dual-columns | Helium | [101]Allenyl-bis-phosphine oxides | [51] | |||
| Leucine | [52] | |||||||||
| Ibuprofen lysine | [53] |
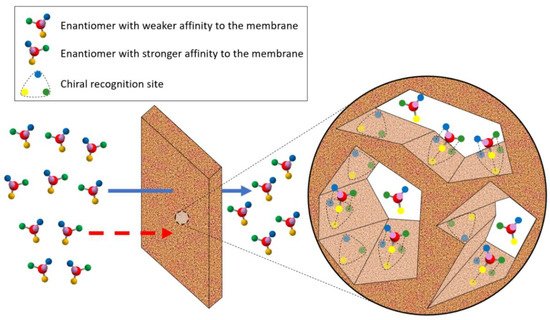
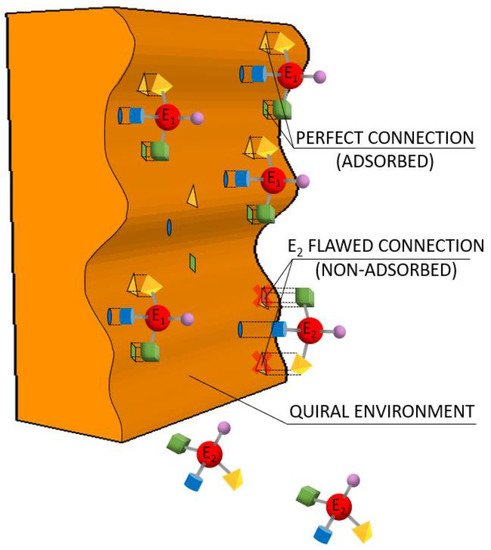
5.2. Membrane
| Enantiomeric pharmaceuticals | |||||||||
| Chirlapak AD and AS, and Chiralcel OD and OJ | |||||||||
| [ | |||||||||
| 138 | |||||||||
| ] | |||||||||
| Tryptophan | Cellulose dialysis membranes | [69] | |||||||
| 2,2-dimethylcyclopropane-carboxamide | g-cyclodextrin | Helium | [102 | 2-phenyl-1-propanol | Glutaraldehyde-crosslinked chitosan membranes | [70] | |||
| Tryptophan | BSA-Immobilized and BSA-Interpenetrating Network Polysulfone Membranes |
[71] | |||||||
| Ketoconazole | Hydrophobic l-isopentyl tartrate and hydrophilic sulfobutylether--cyclodextrin | [72] | |||||||
| ] | |||||||||
| Antiulcer drugs | Chiralpak AD | [139] | 12 amino acids | N-Ethoxycarbonylation was combined with (S)-1-phenylethylamidation | Helium | ||||
| Naproxen | Kromasil CHI-TBB | [140][103] | |||||||
| β-amino acid | CP-Chirasil-Dex CB and CP-Chirasil L-Val | Nitrogen | |||||||
| Warfarin | Chiralpak AD-H | [141[104] | |||||||
| ] | 1-phenylethanol | Permethylated -cyclodextrin | Nitrogen | [105] | |||||
| Chiral sulfoxides | Chiralpak AD | [142] | Amlodipine | Hollow fiber supported liquid membrane | 3-methylhexane, 2,3-dimethylpentane, 3-methyl-heptane, 3,4-dimethylhexane, 2,4-dimethylhexane, 2,3- [73] |
||||
| dimethylhexane, 2,2,3-trimethylpentane | octakis(6-O-methyl-2,3-di-O-pentyl)-g-cyclodextrin | Hydrogen | [106] | ||||||
| Antimycotic azole drugs | Chiralpak AD | [143] | Phenylalanine | Hollow fiber supported liquid membrane | [74] | ||||
| Amino acid derivatives | (l)- or (d)-Valine tert-butylamide grafted on permethylated -cyclodextrin | Helium | |||||||
| Nutlin-3 | Chiralcel OD, Chiralcel AD, Chiralcel OJ, Chirobiotic T, Chirobiotic V | [144][107] | |||||||
| 2,4-dimethylhexane | octakis(6-O-methyl-2,3-di-O-pentyl)-γ-cyclodextrin | Nitrogen | [108] | ||||||
| Phospine-Containing α-Amino Acid Derivatives | Lux Cellulose-1 and -2 | [145] | α- and β-pinene, cis- and trans-pinane, 2,3 butanediol, γ-valerolacton, 1-phenylethyl-lamine, 1-phenylethanol, 2-ethyl-exanoic acid | Derivatized cyclodextrins | |||||
| Acetamide intermediate | Helium | Chiralcel OD-H, Chiralpak AD, Lux Cellulose-2 and Lux Amylose-2[109] | |||||||
| [ | 146] | Methylamphetamine | γ-cyclodextrin | Helium | [110] | ||||
| Tris-(3,5-dimethylphenylcarbamate) of amylose | Chiralcel OD-H and Chiralpak AD-H | [147] | Citronellal, camphor, alanine, leucine, valine, isoleucine, 1-phenyl-1,2-ethandiol, phenylsuccinic acid, and 1-phenyl-ethanol |
Chiral Metal-Organic Frameworks | Nitrogen | [111] | |||
| Cathinone- and amphetamine-related designer drugs | |||||||||
| Mianserin | Chiralcel OJ | [148] | Trifluoroacetyl-l-prolyl chloride | Helium | [112] | ||||
| Chiral fluoro-oxoindole-type compounds | Lux Cellulose-1, Lux Cellulose- | ||||||||
| 1,4-dihydropyridinemonocarboxylic acid | Tert-butylcarbamoylquinine | Methanol and ammonium acetate buffer | [162] | ||||||
| Naringenin and other flavanones | Chiralcel OD-H and Chiralpak AS-H | n-hexane/alcohol | [163] | ||||||
| Piperidine-2,6-dione analogues | Chiralpak IA and Chiralpak IB | Methyl-tert-butyl ether-THF | [164] | ||||||
| Bambuterol | Chiralpak AD | Hexane/2-propanol | [165] | ||||||
| β-Lactams | Cyclodextrin-Based Chiral | Isopropanol-heptane | [166] | 2 and Lux Amylose-2 | [149] | Galaxolide, tonalide, phantolide, traseolide and cashmeran | Chiral heptakis(2,3- di-O-methyl-6-O-t-butyl dimethylsilyl)--cyclodextrin | Helium | [113] |
| Flutriafol | |||||||||
| Ruthenium(II) Polypyridyl Complexes | Cyclodextrin Chiral | Methanol and acetonitrile | [167] | ||||||
| Chiral acids, bases, and amino acids | Zwitterionic ion-exchange-type | Acetic acid, formic acid, diethylamine, and ammonium acetate | [168] | ||||||
| 10 β-adrenergic blockers | CelluCoat column | n-heptane–ethanol–diethylamine | [169] | Chiralpak IA-3 | [ | ||||
| Triazole Fungicides | Chrialcel OD and Chrialcel OJ | Atenolol | Nano-sized chiral imprinted polymers | [75] | |||||
| Ibuprofen | L-tartaric acid derivatives | [76] | |||||||
| DOPA | L-Glutamic acid-Graphene oxide based membranes |
[77] | |||||||
| Tyrosine, phenylalanine and tryptophan | D-penicillamine-modified membrane and N-acetyl-L-cysteine-modified membrane | [78] | |||||||
| Phenylalanine | Regenerated cellulose membranes | [79] | |||||||
| Arginine | Chiral channel protein (FhuAF4) | [80] | |||||||
| Baclofen | Silica-based vancomycin-chiral stationary phase | [81] | |||||||
| Methadone | Chiral (2-hydroxypropyl)-β-cyclodextrin | [82] |
5.3. Chromatography
5.3.1. GC—Gas Chromatography
| Enantiomers | CSP | Gas Carrier | Ref. | ||
|---|---|---|---|---|---|
| β-Blockers | (-)-α-methoxy-α-(trifluoromethyl) phenylacetyl chloride |
||||
| Hexane/2-propanol | |||||
| [ | |||||
| 170 | |||||
| ] | |||||
| 2-arylpropionic | |||||
| acid nonsteroidal anti-infl ammatory drugs | |||||
| Hydroxypropyl-β-cyclodextri | |||||
| Methanol and NaH | |||||
| 2 | PO | 4 buffer | [171] | ||
| 150 | |||||
| ] | |||||
| Citronellal, 1-phenyl-1,2-ethandiol, 1-Phenyl-ethanol, 2-amino-1-butanol, limonene, methionine, proline | |||||
| Porous Chiral Metal-Organic Framework | Nitrogen | ||||
| 4 β-adrenergic blockers | SPE-Chiral | Enantiomeric pharmaceuticals | Chiralpak IC and Chiralpak AD-3 | [151][114] | |
| n-Heptane:ethanol:diethylamine | [ | 2-hexanol, linalool, citronellal, methyl l-b-hydroxyisobutyrate, limonene, rose oxide, dihydrocarvyl acetate, menthol, valine, and leucine |
Metal–Organic Framework on a Chiral Cyclodextrin | Nitrogen | [115] |
| 30 amino acids | Press-Tight© connected Varian-Chrompack Chirasil-l-Val | Helium | [116] |
5.3.2. SFC—Supercritical Fluid Chromatography
Supercritical fluids display transient properties between liquid and gas phases; therefore SFC works as an intermediate between GC and LC [117]. At the supercritical condition, the substances are above its critical temperature and pressure [118][119]. Supercritical fluid was firstly used as an eluent for chromatographic separation in 1962 by Klesper, Iiber and Clark using chlorofluoromethanes at 140 bar and 150–170 °C [120][121]. Supercritical fluids have advantages over liquid and gas states and they are a better solvent due to their higher density in comparison to gas states as they are faster (shorter run times), require lower pressure across column and require lower volume due to its lower viscosity and higher diffusivity over liquid states [118]| 172 |
| Troeger’s base, binaphthol, mandelic methylester, trans-stilbene oxide, flavanone and guaifenesine |
| Chiralcel AD-H and Chiralpak IC |
| [ |
| 152 |
| ] |
| ] | |||
| Ofloxacin | |||
| Ionic liquid-assisted ligand-exchange | |||
| Methanol/water | |||
| [ | |||
| 173 | |||
| ] | |||
| Chiral Pesticides | |||
| Cellulose tris-(3,5-dimethylphenyl-carbamate)-coated chiral | |||
| Ethanol, n-propanol, iso-propanol, n-butanol, and iso-butanol | |||
| [ | 174 | ] | |
| Dihydropyridine derivatives | Polysaccharide-based chiral | Formic acid | [175] |
| Arylpropionic acid derivatives | Chiralpak AD | n-hexane modified either with 2-propanol or ethanol | [176] |
| Illicit drugs | Cyclofructan-based and cyclobond I 2000 RSP | Heptane with ethanol or isopropanol | [177] |
| Ruthenium (II) Polypyridyl Complexes | Cyclofructan | Acetonitrile and methanol | [178] |
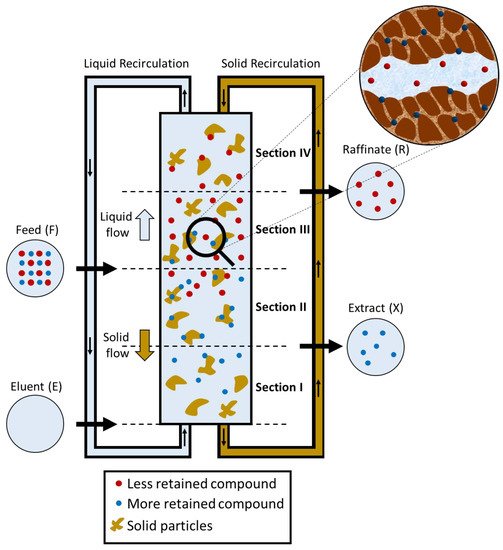
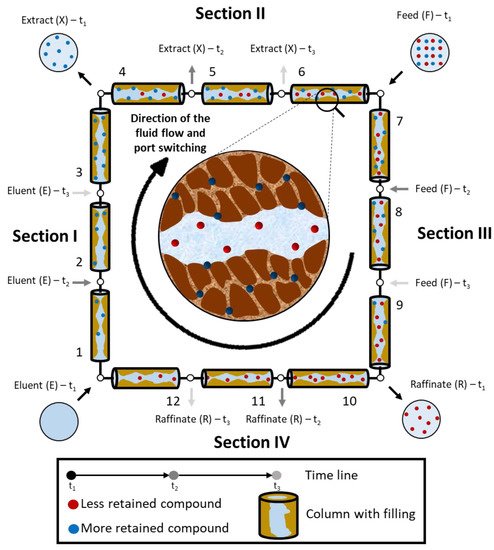
| Section | Function | Illustration | ||||
|---|---|---|---|---|---|---|
| Helium | [ | |||||
| I | 91 | ] | ||||
| The more retained compound moves upward desorbed with the eluent, so that it leaves the system in the Extract stream (X). The eluent cleans the solid that is regenerated prior to being recycled in Section IV. | 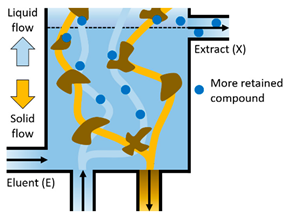 |
β-pinene, sabinene, limonene, linalool, terpinen-4-ol, α-terpineol, linalyl acetate | EtTBS-βCD and DB-5 | Hydrogen | [92] | |
| II | The more retained compound moves downward and is adsorbed on the solid whereas the less retained compound is desorbed with the eluent. This prevents contamination of the less retained compound in the Extract stream (X); the less retained compound moves upward to the Raffinate stream (R). | 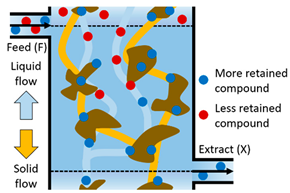 |
Chiral alcohols, chiral sulfoxides, chiral epoxides and acetylated amines |
Chiral ionic liquid stationary phases |
Helium | [93] |
| III | The more retained compound moves downward adsorbed on the solid and the less retained compound is desorbed with the eluent. This prevents contamination in the Raffinate stream (R); the more retained compound moves downward to the Extract steam (X). | 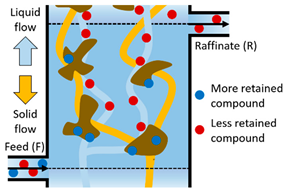 |
Flurbiprofen and ketoprofen | |||
| IV | The less retained compound moves downward adsorbed with the solid flow, so that it leaves the system in the Raffinate stream (R). The solid phase cleans the liquid that is regenerated prior to be recycled to Section I. | 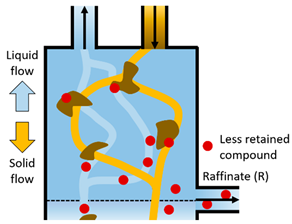 |
A Simulated Moving Bed is a powerful technology for the preparative and analytical scale in laboratory or in industry [83]. SMB chromatography was first presented by UOP (Universal Oil Products) through a United States Patent [4][179][180][181], known as the Sorbex Process, which was designed for oil refining purposes [4]. The technology soon found other industrial applications, such as in biotechnology, pharmaceutical and fine chemistry, as it is a separation system with advantages over batch chromatographic systems and traditional processes (PAIS, 1999). Negawa and Shoji (1992) were the first authors to carry out an enantioresolution in an SMB chromatography, they resolved 1-phenylethanol on CHIRALCEL OD as CSP. Since then, there have been several enantioresolutions reported in the literature, as summarized in Table 11.
| Enantiomers | CSP | Eluent | Ref. |
|---|---|---|---|
| Tramadol | CHIRALPAK AD 20 | 2-propanol, hexane and diethylamine | [182] |
| EMD 53986 | Cellulose-tri-(p-methyl-benzoate) and polymeric silica based | Ethylacetate and ethanol | [183] |
| Guaifenesin, aminoglutetimida, and formoterol | CHIRALCEL OJ and CHIRALCEL OD | Heptane/ethanol | [184] |
| 1,1′-bi-2-naphthol | 3,5-dinitrobenzoyl phenylglycine bonded to silica gel | Heptane and isopropanol | [185] |
| 1,1′-bi-2-naphthol | 3,5-dinitrobenzoyl phenylglycine bonded to silica gel | Heptane and isopropanol | [186] |
| Guaifenesin | CHIRALCEL OD | Heptane/ethanol | [187] |
| 1-phenoxy-2-propanol | CHIRALCEL OD | Hexane and isopropanol | [188] |
| 1,1′-bi-2-naphthol | 3,5-dinitrobenzoyl phenylglycine bonded to silica gel | Heptane and isopropanol | [189] |
| 1-phenyl-1-propanol | CHIRACEL OB | Ethyl acetate and heptane | [190] |
| Trans-stilbene oxide and Tröger’s Base | CHIRALPAK AS and CHIRALPAK AS-V | Hexane/isopropanol | [191] |
| N-carbobenzoxy-tert-leucine and N-Boc-tert-leucine-benzylester | CHIRALCEL OD and CHIRALPAK AD | Heptane/ethanol and Heptane/2-propanol | [192] |
| Phenylpropanolamine | CHIRALPAK AD | Methanol | [193] |
| Guaifenesin | CHIRALCEL OD | Ethanol | [194] |
| Bupivacaine | Kromasil CHI-TBB | Iso-propanol, hexane, and acetic acid | [195] |
| DL-methionine | Eremomycin | Methanol and water | [196] |
| Tröger’s base | CHIRALPAK AD | Methanol | [197] |
| α-Tetralol | CHIRALPAK AD | Heptane/2-propanol | [198] |
| (RS,RS)-2-(2,4-difluorophenyl)butane-1,2,3-triol | CHIRALCEL OJ and CHIRALPAK AD | Hexane, ethanol, and methanol | [199] |
| Tröger’s base | CHIRALPAK AD | Ethanol | [200] |
| Mandelic acid | Kromasil TBB | Hexane and ter-butylmethylether | [201] |
| Tröger’s Base | CHIRALPAK AD | Ethanol | [202] |
| Tröger’s Base | CHIRALPAK AD | Ethanol | [203] |
| Ketoprofen | Chiralpak AD1 | Ethanol, hexane and trifluoroacetic acid | [204] |
| Ketoprofen | Chiralpak AD1 | Ethanol, hexane and trifluoroacetic acid | [205] |
| Guaifenesin | CHIRALCEL OD | Hexane/ethanol | [206] |
| Praziquantel | Chiralcel OZ | Methanol | [207] |
6. Conclusions
References
- Johnson, M. Integrating Health Information: A Case Study of a Health Information Service for Thalidomide Survivors. Inform. Health Soc. Care 2007, 32, 27–33. Molbase. Available online: http://www.molbase.com/ (accessed on 28 January 2019).
- Maier, N.; Pilar, F.; Lindner, W. Separation of Enantiomers: Needs, Challenges, Perspectives. J. Chromatogr. A 2001, 906, 3–33. Johnson, M. Integrating Health Information: A Case Study of a Health Information Service for Thalidomide Survivors. Inform. Health Soc. Care 2007, 32, 27–33.
- Molbase. Available online: http://www.molbase.com/ (accessed on 28 January 2019).Maier, N.; Pilar, F.; Lindner, W. Separation of Enantiomers: Needs, Challenges, Perspectives. J. Chromatogr. A 2001, 906, 3–33.
- Pais, L.M.S. Chiral Separation by Simulated Moving Bed Chromatography; Universidade do Porto: Campo Alegre, Porto, 1999.
- Lough, W.J. Chiral Liquid Chromatography; Springer: New York, NY, USA, 1989.
- CAS. Available online: https://www.cas.org/ (accessed on 24 November 2021).
- Rang, H.; Ritter, J.M.; Flower, R.J.; Henderson, G. Farmacologia; Elsevier: Philadelphia, PA, USA, 2020.
- José, I.; De Veredas, V.; Antônio, M.; Costapinto, C.; José, M.; Carpes, S.; Duarte, R. Cromatografia em leito móvel simulado na produção de substâncias enantiomericamente puras ou enriquecidas em larga escala. Quim. Nova 2006, 29, 1027–1037.
- Aboul-Enein, H.Y.; Wainer, I.W. The Impact of Stereochemistry on Drug Development and Use; Winefordner, J.D., Ed.; Wiley-Interscience: Montreal, QC, Canada, 1997.
- Caldwell, J. Stereochemical Determinants of the Nature and Consequences. J. Chromatogr. A 1995, 694, 39–48.
- Caldwell, J. Importance of Stereospecific Bioanalytical Monitoring in Drug Development. J. Chromatogr. A 1996, 719, 3–13.
- Ariëns, E.J. Stereochemistry: A Source of Problems in Medicinal Chemistry. Med. Res. Rev. 1986, 6, 451–466.
- Gal, J. The Discovery of Stereoselectivity at Biological Receptors: Arnaldo Piutti and the Taste of the Asparagine Enantiomers—History and Analysis on the 125th Anniversary. Eikasmos 2012, 24, 19.
- Mannschreck, A.; Kiesswetter, R.; von Angerer, E. Unequal Activities of Enantiomers via Biological Receptors: Examples of Chiral Drug, Pesticide, and Fragrance Molecules. J. Chem. Educ. 2007, 84, 2012.
- Kurt, J. Histology and Cell Biology, 2nd ed.; Elsevier, Ed.; Harwal Medical Publications: Baltimore, MD, USA, 1991.
- Sanz-Medel, A.; Blanco-González, E. Chiral Trace-Element Speciation in Biological Samples: Present Importance and Application to Speciation for Seleno-Amino Acids. Trends Anal. Chem. 2002, 21, 709–716.
- Ali, I.; Alam, S.D.; Al-Othman, Z.A.; Farooqi, J.A. Recent Advances in SPE-Chiral-HPLC Methods for Enantiomeric Separation of Chiral Drugs in Biological Samples. J. Chromatogr. Sci. 2013, 51, 645–654.
- Bhupinder Singh Sekhon. Enantioseparation of Chiral Drugs—An Overview. Int. J. PharmTech Res. 2010, 2, 1584–1594.
- Erb, S. Single-Enantiomer Drugs Poised for Further Market Growth. Pharm. Technol. 2006, 30, s14–s18.
- Technology Catalysts. Available online: https://technology-catalysts.com/industry-expertise/fine/ (accessed on 29 January 2019).
- De Camp, W.H. The FDA Perspective on the Development of Stereoisomers. Chirality 1989, 1, 2–6.
- FDA. FDA’S Policy Statement for the Development of New Stereoisomeric Drugs; FDA: Silver Spring, MD, USA, 1992; Volume 4.
- Rekoske, J.E. Chiral Separations I Figure I. Distrib. Drugs Dev. Worldw. 2001, 47, 2–5.
- Rouhi, A.M. Fine Chemicals Companies Are Jockeying for Position to Deliver the Increasingly Complicated Chiral Small Molecules of the Future. Chem. Eng. News 2003, 81, 45–61.
- Pharmacompass. Available online: https://www.pharmacompass.com/radio-compass-blog/top-drugs-by-sales-in-2017-who-sold-the-blockbuster-drugs (accessed on 19 December 2021).
- Hajos, Z.G.; Parrish, D.R. Asymmetric Synthesis of Bicyclic Intermediates of Natural Product Chemistry. J. Org. Chem. 1974, 39, 1615–1621.
- Keith, J.M.; Larrow, J.F.; Jacobsen, E.N. Practical Considerations in Kinetic Resolution Reactions. Adv. Synth. Catal. 2001, 343, 5–26.
- Trost, B.M. Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281.
- Carvalho, P.O.; Cass, Q.B.; Calafatti, S.A.; Contesini, F.J.; Bizaco, R. Review-Alternatives for the Separation of Drug Enantiomers: Ibuprofen as a Model Compound. Braz. J. Chem. Eng. 2006, 23, 291–300.
- Dalgliesh, C.E. The Optical Resolution of Aromatic Amino-Acids on Paper Chromatograms. J. Chem. Soc. 1952, 3, 756.
- Afonso, C.A.M.; Crespo, J.G. Recent Advances in Chiral Resolution through Membrane-Based Approaches. Angew. Chem. Int. Ed. Engl. 2004, 43, 5293–5295.
- Pirkle, W.H.; Pochapsky, T.C. Considerations of Chiral Recognition Relevant to the Liquid Chromatographic Separation of Enantiomers. Chem. Rev. 1989, 89, 347–362.
- Singh, K.; Ingole, P.G.; Bajaj, H.C.; Gupta, H. Preparation, Characterization and Application of β-Cyclodextrin-Glutaraldehyde Crosslinked Membrane for the Enantiomeric Separation of Amino Acids. Desalination 2012, 298, 13–21.
- Francotte, E.R. Enantioselective Chromatography as a Powerful Alternative for the Preparation of Drug Enantiomers. J. Chromatogr. A 2001, 906, 379–397.
- Ward, T.J.; Ward, K.D. Chiral Separations: A Review of Current Topics and Trends. Anal. Chem. 2012, 84, 626–635.
- Adrjanowicz, K.; Kaminski, K.; Paluch, M.; Niss, K. Crystallization Behavior and Relaxation Dynamics of Supercooled S-Ketoprofen and the Racemic Mixture along an Isochrone. Cryst. Growth Des. 2015, 15, 3257–3263.
- Collet, A.; Brienne, M.J.; Jacques, J. Optical Resolution by Direct Crystallization of Enantiomer Mixtures. Chem. Rev. 1980, 80, 215–230.
- Lorenz, H.; Seidel-Morgenstern, A. Processes to Separate Enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250.
- Gervais, C.; Beilles, S.; Cardinaël, P.; Petit, S.; Coquerel, G. Oscillating Crystallization in Solution between (+)- and (-)-5-Ethyl-5-Methylhydantoin under the Influence of Stirring. J. Phys. Chem. B 2002, 106, 646–652.
- Xie, R.; Chu, L.Y.; Deng, J.G. Membranes and Membrane Processes for Chiral Resolution. Chem. Soc. Rev. 2008, 37, 1243–1263.
- Collet, A. Resolution of Racemates: Did You Say “Classical”? Angew. Chem. Int. Ed. 1998, 37, 3239–3241.
- Rodrigo, A.A.; Lorenz, H.; Seidel-Morgenstern, A. Online Monitoring of Preferential Crystallization of Enantiomers. Chirality 2004, 16, 499–508.
- Viedma, C. Enantiomeric Crystallization from DL-Aspartic and DL-Glutamic Acids. Implic. Biomol. Chirality Orig. Life 2001, 31, 501–509.
- Ge, S.H.; Li, X.G.; Hsing, I.M. Water Management in PEMFCs Using Absorbent Wicks. J. Electrochem. Soc. 2004, 151, B523.
- Kmecz, I.; Simándi, B.; Székely, E.; Fogassy, E. Resolution of N-Methylamphetamine Enantiomers with Tartaric Acid Derivatives by Supercritical Fluid Extraction. Tetrahedron Asymmetry 2004, 15, 1841–1845.
- Elsner, M.P.; Menéndez, D.F.; Muslera, E.A.; Seidel-Morgenstern, A. Experimental Study and Simplified Mathematical Description of Preferential Crystallization. Chirality 2005, 17, 183–195.
- Lorenz, H.D.P.; Seidel-Morgenstern, A. Application of Preferential Crystallization to Resolve Racemic Compounds in a Hybrid Process. Chirality 2006, 18, 828–840.
- Medina, D.D.; Goldshtein, J.; Margel, S.; Mastai, Y. Enantioselective Crystallization on Chiral Polymeric Microspheres. Adv. Funct. Mater. 2007, 17, 944–950.
- D’Oria, E.; Karamertzanis, P.G.; Price, S.L. Spontaneous Resolution of Enantiomers by Crystallization: Insights from Computed Crystal Energy Landscapes. Cryst. Growth Des. 2010, 10, 1749–1756.
- Springuel, G.; Leyssens, T. Innovative Chiral Resolution Using Enantiospecific Co-Crystallization in Solution. Cryst. Growth Des. 2012, 12, 3374–3378.
- Gangadhararao, G.; Tulichala, R.N.P.; Swamy, K.C.K. Spontaneous Resolution upon Crystallization of Allenyl-Bis-Phosphine Oxides. Chem. Commun. 2015, 51, 7168–7171.
- Manoj, K.; Takahashi, H.; Morita, Y.; Gonnade, R.G.; Iwama, S.; Tsue, H.; Tamura, R. Preferential Enrichment of DL-Leucine Using Cocrystal Formation With Oxalic Acid Under Nonequilibrium Crystallization Conditions. Chirality 2015, 27, 405–410.
- Simon, M.; Donnellan, P.; Glennon, B.; Jones, R.C. Resolution via Diastereomeric Salt Crystallization of Ibuprofen Lysine: Ternary Phase Diagram Studies. Chem. Eng. Technol. 2018, 41, 921–927.
- Rougeot, C.; Hein, J.E. Application of Continuous Preferential Crystallization to Efficiently Access Enantiopure Chemicals. Org. Process. Res. Dev. 2015, 19, 1809–1819.
- Fogassy, E.; Nógrádi, M.; Kozma, D.; Egri, G.; Pálovics, E.; Kiss, V. Optical Resolution Methods. Org. Biomol. Chem. 2006, 4, 3011–3030.
- Van Der Ent, E.M.; Van Riet, K.; Keurentjes, J.T.F.; Van Der Padt, A. Design Criteria for Dense Permeation-Selective Membranes for Enantiomer Separations. J. Membr. Sci. 2001, 185, 207–221.
- Higuchi, A.; Higuchi, Y.; Furuta, K.; Yoon, B.O.; Hara, M.; Maniwa, S.; Saitoh, M.; Sanui, K. Chiral Separation of Phenylalanine by Ultrafiltration through Immobilized DNA Membranes. J. Memb. Sci. 2003, 221, 207–218.
- Matsuoka, Y.; Kanda, N.; Lee, Y.M.; Higuchi, A. Chiral Separation of Phenylalanine in Ultrafiltration through DNA-Immobilized Chitosan Membranes. J. Memb. Sci. 2006, 280, 116–123.
- Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation. Symmetry 2017, 9, 206.
- Kaner, R.B. Gas, Liquid and Enantiomeric Separations Using Polyaniline. Synth. Met. 2001, 125, 65–71.
- Thoelen, C.; De Bruyn, M.; Theunissen, E.; Kondo, Y.; Vankelecom, I.F.J.; Grobet, P.; Yoshikawa, M.; Jacobs, P.A. Membranes Based on Poly(γ-Methyl-L-Glutamate): Synthesis, Characterization and Use in Chiral Separations. J. Memb. Sci. 2001, 186, 153–163.
- Hadik, P.; Szabó, L.P.; Nagy, E. D,L-Lactic Acid and D,L-Alanine Enantioseparation by Membrane Process. Desalination 2002, 148, 193–198.
- Donato, L.; Figoli, A.; Drioli, E. Novel Composite Poly(4-Vinylpyridine)/Polypropylene Membranes with Recognition Properties for (S)-Naproxen. J. Pharm. Biomed. Anal. 2005, 37, 1003–1008.
- Gumí, T.; Valiente, M.; Palet, C. Elucidation of SR-Propranolol Transport Rate and Enantioselectivity through Chiral Activated Membranes. J. Memb. Sci. 2005, 256, 150–157.
- Higuchi, A.; Hayashi, A.; Kanda, N.; Sanui, K.; Kitamura, H. Chiral Separation of Amino Acids in Ultrafiltration through DNA-Immobilized Cellulose Membranes. J. Mol. Struct. 2005, 739, 145–152.
- Ghazali, N.F.; Ferreira, F.C.; White, A.J.P.; Livingston, A.G. Enantiomer Separation by Enantioselective Inclusion Complexation-Organic Solvent Nanofiltration. Tetrahedron Asymmetry 2006, 17, 1846–1852.
- Maximini, A.; Chmiel, H.; Holdik, H.; Maier, N.W. Development of a Supported Liquid Membrane Process for Separating Enantiomers of N-Protected Amino Acid Derivatives. J. Memb. Sci. 2006, 276, 221–231.
- Wang, H.D.; Chu, L.Y.; Song, H.; Yang, J.P.; Xie, R.; Yang, M. Preparation and Enantiomer Separation Characteristics of Chitosan/β-Cyclodextrin Composite Membranes. J. Memb. Sci. 2007, 29, 262–270.
- Xiao, Y.; Chung, T.S. Functionalization of Cellulose Dialysis Membranes for Chiral Separation Using Beta-Cyclodextrin Immobilization. J. Memb. Sci. 2007, 290, 78–85.
- Xiong, W.W.; Wang, W.F.; Zhao, L.; Song, Q.; Yuan, L.M. Chiral Separation of (R,S)-2-Phenyl-1-Propanol through Glutaraldehyde-Crosslinked Chitosan Membranes. J. Memb. Sci. 2009, 328, 268–272.
- Singh, K.; Bajaj, H.C.; Ingole, P.; Bhattacharya, A. Comparative Study of Enantioseparation of Racemic Tryptophan by Ultrafiltration Using BSA-Immobilized and BSA-Interpenetrating Network Polysulfone Membranes. Sep. Sci. Technol. 2010, 45, 346–354.
- Wang, Z.; Cai, C.; Lin, Y.; Bian, Y.; Guo, H.; Chen, X. Enantioselective Separation of Ketoconazole Enantiomers by Membrane Extraction. Sep. Purif. Technol. 2011, 79, 63–71.
- Sunsandee, N.; Leepipatpiboon, N.; Ramakul, P.; Pancharoen, U. The Selective Separation of (S)-Amlodipine via a Hollow Fiber Supported Liquid Membrane: Modeling and Experimental Verification. Chem. Eng. J. 2012, 180, 299–308.
- Naksang, C.; Sunsandee, N.; Thamphiphit, N.; Pancharoen, U.; Ramakul, P.; Leepipatpiboon, N. Synergistic Enantioseparation of Rac-Phenylalanine via Hollow Fiber Supported Liquid Membrane. Sep. Sci. Technol. 2013, 48, 867–876.
- Alizadeh, T. Synthesis of a Nano-Sized Chiral Imprinted Polymer and Its Use as an (S)-Atenolol Carrier in the Bulk Liquid Membrane. J. Sep. Sci. 2014, 37, 1887–1895.
- Zhang, F.; He, L.; Sun, W.; Cheng, Y.; Liu, J.; Ren, Z. Chiral Liquid Membrane for Enantioselective Separation of Racemic Ibuprofen by L-Tartaric Acid Derivatives. RSC Adv. 2015, 5, 41729–41735.
- Meng, C.; Sheng, Y.; Chen, Q.; Tan, H.; Liu, H. Exceptional Chiral Separation of Amino Acid Modified Graphene Oxide Membranes with High-Flux. J. Memb. Sci. 2017, 526, 25–31.
- Huang, X.Y.; Pei, D.; Liu, J.F.; Di, D.L. A Review on Chiral Separation by Counter-Current Chromatography: Development, Applications and Future Outlook. J. Chromatogr. A 2018, 1531, 1–12.
- Keating, J.J.; Bhattacharya, S.; Belfort, G. Separation of D, L-Amino Acids Using Ligand Exchange Membranes. J. Memb. Sci. 2018, 555, 30–37.
- Anand, D.; Dhoke, G.V.; Gehrmann, J.; Garakani, T.M.; Davari, M.D.; Bocola, M.; Zhu, L.; Schwaneberg, U. Chiral Separation of d/l-Arginine with Whole Cells through an Engineered FhuA Nanochannel. Chem. Commun. 2019, 55, 5431–5434.
- D’Orazio, G.; Fanali, C.; Gentili, A.; Tagliaro, F.; Fanali, S. Nano-Liquid Chromatography for Enantiomers Separation of Baclofen by Using Vancomycin Silica Stationary Phase. J. Chromatogr. A 2019, 1605, 6–13.
- Hadjmohammadi, M.R.; Hashemi, M. Chiral Separation of Methadone Using Solid Membrane Extraction Based on Chiral Selector, Solid Membrane: Sheep Skin Leather. J. Iran. Chem. Soc. 2019, 16, 1611–1616.
- Rodrigues, A.E.; Pereira, C.; Minceva, M.; Pais, L.S.; Ribeiro, A.M.; Ribeiro, A.; Silva, M.; Graça, N.; Santos, J.C. Simulated Moving Bed Technology: Principles, Design and Process Applications; Joe Hayton: Amsterdam, The Netherlands, 2015.
- Audebert, R. Direct Resolution of Enantiomers in Column Liouid Chromatography. J. Liq. Chromatogr. 1979, 2, 1063–1095.
- Gil-Av, E.; Feibush, B.; Charles-Sigler, R. Separation of Enantiomers by Gas Liquid Chrimatography with an Optically Active Stationary Phase. Br. J. Psychiatry 1966, 10, 1009–1015.
- He, L.; Beesley, T.E. Applications of Enantiomeric Gas Chromatography: A Review. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1075–1114.
- Zhang, Y.; Wu, D.R.; Wang-Iverson, D.B.; Tymiak, A.A. Enantioselective Chromatography in Drug Discovery. Drug Discov. Today 2005, 10, 571–577.
- Schurig, V. Enantiomer Analysis by Complexation Gas Chromatography. J. Chromatogr. A 1988, 441, 135–153.
- Schurig, V.; Kreidler, D. Gas-Chromatographic Enantioseparation of Unfunctionalized Chiral Hydrocarbons: An Overview. Methods Mol. Biol. 2013, 970, 45–67.
- Lang, J.C.; Armstrong, D.W. Chiral Surfaces: The Many Faces of Chiral Recognition. Curr. Opin. Colloid Interface Sci. 2017, 32, 94–107.
- Kim, K.H.; Lee, J.H.; Ko, M.Y.; Hong, S.P.; Youm, J.R. Chiral Separation of β-Blockers after Derivatization with (-)-AMethoxy-α-(Trifluoromethyl)Phenylacetyl Chloride by Gas Chromatography. Arch. Pharm. Res. 2001, 24, 402–406.
- Shellie, R.; Marriott, P.J. Comprehensive Two-Dimensional Gas Chromatography with Fast Enantioseparation. Anal. Chem. 2002, 74, 5426–5430.
- Ding, J.; Welton, T.; Armstrong, D.W. Chiral Ionic Liquids as Stationary Phases in Gas Chromatography. Anal. Chem. 2004, 76, 6819–6822.
- Paik, M.J.; Nguyen, D.T.; Kim, K.R. Enantioseparation of Flurbiprofen and Ketoprofen in Patches and in Urine Excretions by Achiral Gas Chromatography. Arch. Pharm. Res. 2004, 27, 1295–1301.
- Pätzold, R.; Schieber, A.; Brückner, H. Gas Chromatographic Quantification of Free D-Amino Acids in Higher Vertebrates. Biomed. Chromatogr. 2005, 19, 466–473.
- Petrović, M.; Debeljak, Ž.; Blažević, N. Optimization of Gas Chromatographic Method for the Enantioseparation of Arylpropionic Non-Steroidal Anti-Inflammatory Drug Methyl Esters. J. Pharm. Biomed. Anal. 2005, 39, 531–534.
- Shi, X.; Guo, H.; Wang, M. Enantioseparation of Chiral Epoxides Using Four New Cyclodextrin Derivatives as Chiral Stationary Phases of Capillary Gas Chromatography. Anal. Chim. Acta 2005, 553, 43–49.
- Sicoli, G.; Jiang, Z.; Jicsinsky, L.; Schurig, V. Modified Linear Dextrins (“acyclodextrins”) as New Chiral Selectors for the Gas-Chromatographic Separation of Enantiomers. Angew. Chem. Int. Ed. 2005, 44, 4092–4095.
- Takahisa, E.; Engel, K.H. 2,3-Di-O-Methoxymethyl-6-O-Tert-Butyldimethylsilyl-γ-Cyclodextrin: A New Class of Cyclodextrin Derivatives for Gas Chromatographic Separation of Enantiomers. J. Chromatogr. A 2005, 1063, 181–192.
- Levkin, P.A.; Ruderisch, A.; Schurig, V. Combining the Enantioselectivity of a Cyclodextrin and a Diamide Selector in a Mixed Binary Gas-Chromatographic Chiral Stationary Phase. Chirality 2006, 18, 49–63.
- Paik, M.J.; Nguyen, D.T.; Kim, K.R. N-Menthoxycarbonylation Combined with Trimethylsilylation for Enantioseparation of β-Blockers by Achiral Dual-Column Gas Chromatography. J. Chromatogr. A 2006, 1103, 177–181.
- Zheng, R.C.; Zheng, Y.G.; Shen, Y.C. Enantioseparation and Determination of 2,2-Dimethylcyclopropanecarboxamide and Corresponding Acid in the Bioconversion Broth by Gas Chromatography. Biomed. Chromatogr. 2007, 21, 610–615.
- Paik, M.J.; Lee, J.; Kim, K.R. N-Ethoxycarbonylation Combined with (S)-1-Phenylethylamidation for Enantioseparation of Amino Acids by Achiral Gas Chromatography and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2008, 1214, 151–156.
- Forró, E. New Gas Chromatographic Method for the Enantioseparation of β-Amino Acids by a Rapid Double Derivatization Technique. J. Chromatogr. A 2009, 1216, 1025–1029.
- Grisales, J.O.; Lebed, P.J.; Keunchkarian, S.; González, F.R.; Castells, C.B. Permethylated β-Cyclodextrin in Liquid Poly(Oxyethylene) as a Stationary Phase for Capillary Gas Chromatography. J. Chromatogr. A 2009, 1216, 6844–6851.
- Sicoli, G.; Kreidler, D.; Czesla, H.; Hopf, H.; Schurig, V. Gas Chromatographic Enantioseparation of Unfunctionalized Chiral Alkanes: A Challenge in Separation Science (Overview, State of the Art, and Perspectives). Chirality 2009, 21, 182–198.
- Stephany, O.; Dron, F.; Tisse, S.; Martinez, A.; Nuzillard, J.M.; Peulon-Agasse, V.; Cardinaël, P.; Bouillon, J.P. (L)- or (d)-Valine Tert-Butylamide Grafted on Permethylated β-Cyclodextrin Derivatives as New Mixed Binary Chiral Selectors. Versatile Tools for Capillary Gas Chromatographic Enantioseparation. J. Chromatogr. A 2009, 1216, 4051–4062.
- Kühnle, M.; Kreidler, D.; Holtin, K.; Czesla, H.; Schuler, P.; Schurig, V.; Albert, K. Online Coupling of Enantioselective Capillary Gas Chromatography with Proton Nuclear Magnetic Resonance Spectroscopy. Chirality 2010, 22, 808–812.
- Schurig, V. Utilisation Des Cyclodextrines Dérivées Comme Sélecteurs de Séparation Énantiomérique Par Chromatographie Gazeuse. Ann. Pharm. Fr. 2010, 68, 82–98.
- Drake, S.; Morrison, C.; Smith, F. Simultaneous Chiral Separation of Methylamphetamine and Common Precursors Using Gas Chromatography/Mass Spectrometry. Chirality 2011, 23, 593–601.
- Xie, S.M.; Zhang, Z.J.; Wng, Z.Y.; Yuan, L.M. Chiral Metal-Organic Frameworks for High-Resolution Gas Chromatographic Separations. J. Am. Chem. Soc. 2011, 133, 11892–11895.
- Mohr, S.; Weiß, J.A.; Spreitz, J.; Schmid, M.G. Chiral Separation of New Cathinone- and Amphetamine-Related Designer Drugs by Gas Chromatography-Mass Spectrometry Using Trifluoroacetyl-l-Prolyl Chloride as Chiral Derivatization Reagent. J. Chromatogr. A 2012, 1269, 352–359.
- Wang, L.; McDonald, J.A.; Khan, S.J. Enantiomeric Analysis of Polycyclic Musks in Water by Chiral Gas Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1303, 66–75.
- Xie, S.M.; Zhang, X.H.; Zhang, Z.J.; Yuan, L.M. Porous Chiral Metal-Organic Framework InH(D-C10H14O4)2 with Anionic-Type Diamond Network for High-Resolution Gas Chromatographic Enantioseparations. Anal. Lett. 2013, 46, 753–763.
- Liu, H.; Xie, S.M.; Ai, P.; Zhang, J.H.; Zhang, M.; Yuan, L.M. Metal-Organic Framework Co(D-Cam)1/2(Bdc)1/2(Tmdpy) for Improved Enantioseparations on a Chiral Cyclodextrin Stationary Phase in Gas Chromatography. Chempluschem 2014, 79, 1103–1108.
- Myrgorodska, I.; Meinert, C.; Martins, Z.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Quantitative Enantioseparation of Amino Acids by Comprehensive Two-Dimensional Gas Chromatography Applied to Non-Terrestrial Samples. J. Chromatogr. A 2016, 1433, 131–136.
- Henry, M.C.; Road, G.S.; Prussia, K.; Yonker, C.R.; Pacific, B.; National, N.; Box, L.P.O.; Richland, K. Supercritical Fluid Chromatography, Pressurized Liquid Extraction, and Supercritical Fluid Extraction. J. Chromatogr. A 2006, 78, 3909–3916.
- De Klerck, K.; Mangelings, D.; Vander Heyden, Y. Supercritical Fluid Chromatography for the Enantioseparation of Pharmaceuticals. J. Pharm. Biomed. Anal. 2012, 69, 77–92.
- Miller, L. Preparative Enantioseparations Using Supercritical Fluid Chromatography. J. Chromatogr. A 2012, 1250, 250–255.
- Klesper, E.; Iber, K.; Clark, M. High Pressure Gas Chromatography above Critical Temperatures. J. Org. Chem. 1962, 27, 700–706.
- Taylor, L.T. Supercritical Fluid Chromatography for the 21st Century. J. Supercrit. Fluids 2009, 47, 566–573.
- Pasquali, I.; Bettini, R. Are Pharmaceutics Really Going Supercritical? Int. J. Pharm. 2008, 364, 176–187.
- Chester, T.L.; Pinkston, J.D.; Raynie, D.E. Supercritical Fluid Chromatography and Extraction. Anal. Chem. 1994, 66, 106–130.
- Williams, K.L.; Sander, L.C. Enantiomer Separations on Chiral Stationary Phases in Supercritical Fluid Chromatography. J. Chromatogr. A 1997, 785, 149–158.
- Mourier, P.A.; Eliot, E.; Caude, M.H.; Rosset, R.H.; Bouchet, L. Supercritical and Subcritical Fluid Chromatography on a Chiral Stationary Phase for the Resolution of Phosphine Oxide Enantiomers. Anal. Chem. 1985, 8, 2819–2823.
- Macaudière, P.; Caude, M.; Rosset, R. Chiral Resolutions in SFC: Mechanisms and Applications with Various Chiral Stationary Phases. J. Chromatogr. Sci. 1989, 27, 583–591.
- Stringham, R.W.; Lynam, K.G.; Grasso, C.C. Application of Subcritical Fluid Chromatography to Rapid Chiral Method Development. Anal. Chem. 1994, 66, 1949–1954.
- Johannsen, M. Separation of Enantiomers of Ibuprofen on Chiral Stationary Phases by Packed Column Supercritical Fluid Chromatography. J. Chromatogr. A 2001, 937, 135–138.
- Toribio, L.; Bernal, J.L.; Nozal, M.J.; Jimenez, J.J.; Nieto, E.M. Applications of the Chiralpak AD and Chiralcel OD Chiral Columns in the Enantiomeric Separation of Several Dioxolane Compounds by Supercritical Fluid Chromatography Q. J. Chromatogr. A 2001, 921, 305–313.
- Wang, T.; Barber, M.; Hardt, I.; Kassel, D.B. Mass-Directed Fractionation and Isolation of Pharmaceutical Compounds by Packed-Column Supercritical Fluid Chromatography/Mass Spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 2067–2075.
- Garzotti, M.; Hamdan, M. Supercritical Fluid Chromatography Coupled to Electrospray Mass Spectrometry: A Powerful Tool for the Analysis of Chiral Mixtures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 770, 53–61.
- Liu, Y.; Berthod, A.; Mitchell, C.R.; Xiao, T.L.; Zhang, B.; Armstrong, D.W. Super/Subcritical Fluid Chromatography Chiral Separations with Macrocyclic Glycopeptide Stationary Phases. J. Chromatogr. A 2002, 978, 185–204.
- Nozal, M.J.; Toribio, L.; Bernal, J.L.; Nieto, E.M.; Jime, J.J. Separation of Albendazole Sulfoxide Enantiomers by Chiral Supercritical-Fluid Chromatography. J. Biochem. Biophys. Methods 2002, 54, 339–345.
- Nozal, M.J.; Toribio, L.; Bernal, J.L.; Castano, N.S. Eparation of Triadimefon and Triadimenol Enantiomers and Diastereoisomers by Supercritical Fluid Chromatography. J. Chromatogr. A. 2003, 986, 135–141.
- Toribio, L.; Nozal, M.J.; Bernal, J.L.; Nieto, E.M. Use of Semipreparative Supercritical Fluid Chromatography to Obtain Small Quantities of the Albendazole Sulfoxide Enantiomers. J. Chromatogr. A 2003, 1011, 155–161.
- Toribio, L.; Nozal, M.J.; Bernal, J.L.; Jimenez, J.J.; Alonso, C. Chiral Separation Ofsome Triazole Pesticides by Supercritical Fluid Chromatography. J. Chromatogr. A 2004, 1046, 249–253.
- del Nozal, M.J.; Toribio, L.; Bernal, J.L.; Alonso, C.; Jiménez, J.J. Chiral Separation of Omeprazole and Several Related Benzimidazoles Using Supercritical Fluid Chromatography. J. Sep. Sci. 2004, 27, 1023–1029.
- Maftouh, M.; Granier-loyaux, C.; Chavana, E.; Marini, J.; Pradines, A.; Vander, Y.; Picard, C. Screening Approach for Chiral Separation of Pharmaceuticals Part III. Supercritical Fluid Chromatography for Analysis and Purification in Drug Discovery. J. Chromatogr. A 2005, 1088, 67–81.
- Toribio, L.; Del Nozal, M.J.; Bernal, J.L.; Alonso, C.; Jiménez, J.J. Comparative Study of the Enantioselective Separation of Several Antiulcer Drugs by High-Performance Liquid Chromatography and Supercritical Fluid Chromatography. J. Chromatogr. A 2005, 1091, 118–123.
- Yang, Y.; Su, B.; Yan, Q.; Ren, Q. Separation of Naproxen Enantiomers by Supercritical/Subcritical Fluid Chromatography. J. Pharm. Biomed. Anal. 2005, 39, 815–818.
- Coe, R.A.; Rathe, J.O.; Lee, J.W. Supercritical Fluid Chromatography-Tandem Mass Spectrometry for Fast Bioanalysis of R/S-Warfarin in Human Plasma. J. Pharm. Biomed. Anal. 2006, 42, 573–580.
- Toribio, L.; Alonso, C.; del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J. Enantiomeric Separation of Chiral Sulfoxides by Supercritical Fluid Chromatography. J. Sep. Sci. 2006, 29, 1363–1372.
- Toribio, L.; del Nozal, M.J.; Bernal, J.L.; Alonso, C.; Jiménez, J.J. Enantiomeric Separation of Several Antimycotic Azole Drugs Using Supercritical Fluid Chromatography. J. Chromatogr. A 2007, 1144, 255–261.
- Wang, Z.; Jonca, M.; Lambros, T.; Ferguson, S.; Goodnow, R. Exploration of Liquid and Supercritical Fluid Chromatographic Chiral Separation and Purification of Nutlin-3-A Small Molecule Antagonist of MDM2. J. Pharm. Biomed. Anal. 2007, 45, 720–729.
- West, C.; Bouet, A.; Gillaizeau, I.; Coudert, G.; Lafosse, M.; Lesellier, E. Chiral Separation of Phospine-Containing a -Amino Acid Derivatives Using Two Complementary Cellulosic Stationary Phases in Supercritical Fluid Chromatography. Chirality 2010, 251, 242–251.
- Toribio, L.; Nozal, M.J.; Bernal, J.L.; Bernal, J.; Martín, M.T. Study of the Enantiomeric Separation of an Acetamide Intermediate by Using Supercritical Fluid Chromatography and Several Polysaccharide Based Chiral Stationary Phases. J. Chromatogr. A 2011, 1218, 4886–4891.
- West, C.; Guenegou, G.; Zhang, Y.; Morin-Allory, L. Insights into Chiral Recognition Mechanisms in Supercritical Fluid Chromatography. II. Factors Contributing to Enantiomer Separation on Tris-(3,5-Dimethylphenylcarbamate) of Amylose and Cellulose Stationary Phases. J. Chromatogr. A 2011, 1218, 2033–2057.
- Hamman, C.; Schmidt, D.E.; Wong, M.; Hayes, M. The Use of Ammonium Hydroxide as an Additive in Supercritical Fluid Chromatography for Achiral and Chiral Separations and Purifications of Small, Basic Medicinal Molecules. J. Chromatogr. A 2012, 1218, 7886–7894.
- West, C.; Bouet, A.; Routier, S.; Lesellier, E. Effects of Mobile Phase Composition and Temperature on the Supercritical Fluid Chromatography Enantioseparation of Chiral Fluoro-Oxoindole-Type Compounds with Chlorinated Polysaccharide Stationary Phases. J. Chromatogr. A 2012, 1269, 325–335.
- Tao, Y.; Dong, F.; Xu, J.; Liu, X.; Cheng, Y.; Liu, N.; Chen, Z.; Zheng, Y. Green and Sensitive Supercritical Fluid Chromatographic-Tandem Mass Spectrometric Method for the Separation and Determination of Flutriafol Enantiomers in Vegetables, Fruits, and Soil. J. Agric. Food Chem. 2014, 62, 11457–11464.
- Regalado, E.L.; Welch, C.J. Pushing the Speed Limit in Enantioselective Supercritical Fluid Chromatography. J. Sep. Sci. 2015, 38, 2826–2832.
- Khater, S.; Lozac’h, M.A.; Adam, I.; Francotte, E.; West, C. Comparison of Liquid and Supercritical Fluid Chromatography Mobile Phases for Enantioselective Separations on Polysaccharide Stationary Phases. J. Chromatogr. A 2016, 1467, 463–472.
- Jung, M.; Schurig, V. Extending the Scope of Enantiomer Separation by Capillary Supercritical Fluid Chromatography on Immobilized Poly. J. High. Resolut. Chromatogr. 1993, 16, 215–223.
- Armstrong, D.W. Optical Isomer Separation by Liquid Chromatography. Anal. Chem. 1987, 59, 84A–97A.
- Däppen, R.; Arm, H.; Meyer, V.R. Applications and Limitations of Commercially Available Chiral Stationary Phases for High-Performance Liquid Chromatography. J. Chromatogr. A 1986, 373, 1–20.
- Pham-Huy, C.; Villain-Pautet, G.; Hua, H.; Chikhi-Chorfi, N.; Galons, H.; Thevenin, M.; Claude, J.R.; Warnet, J.M. Separation of Oxazepam, Lorazepam, and Temazepam Enantiomers by HPLC on a Derivatized Cyclodextrin-Bonded Phase: Application to the Determination of Oxazepam in Plasma. J. Biochem. Biophys. Methods 2002, 54, 287–299.
- Boatto, G.; Nieddu, M.; Faedda, M.V.; De Caprariis, P. Enantiomeric Separation by HPLC of 1,4-Dihydropyridines with Vancomycin as Chiral Selector. Chirality 2003, 15, 494–497.
- Narayana, C.; Suresh, T.; Mahender Rao, S.; Dubey, P.K.; Moses Babu, J. A Validated Chiral HPLC Method for the Enantiomeric Separation of Linezolid on Amylose Based Stationary Phase. J. Pharm. Biomed. Anal. 2003, 32, 21–28.
- Putkonen, T.; Tolvanen, A.; Jokela, R.; Caccamese, S.; Parrinello, N. Total Synthesis of (±)-Tangutorine and Chiral HPLC Separation of Enantiomers. Tetrahedron 2003, 59, 8589–8595.
- Zhang, X.; Ouyang, J.; Baeyens, W.R.G.; Zhai, S.; Yang, Y.; Huang, G. Enantiomeric Separation of β-Blockers by HPLC Using (R)-1-Naphthylglycine and 3,5-Dinitrobenzoic Acid as Chiral Stationary Phase. J. Pharm. Biomed. Anal. 2003, 31, 1047–1057.
- Kumar, Y.R.; Ramulu, G.; Vevakanand, V.V.; Vaidyanathan, G.; Srinivas, K.; Kumar, M.K.; Mukkanti, K.; Reddy, M.S.; Venkatraman, S.; Suryanarayana, M.V. A Validated Chiral HPLC Method for the Enantiomeric Separation of Tolterodine Tartarate. J. Pharm. Biomed. Anal. 2004, 35, 1279–1285.
- Lämmerhofer, M.; Gyllenhaal, O.; Lindner, W. HPLC Enantiomer Separation of a Chiral 1,4-Dihydropyridine Monocarboxylic Acid. J. Pharm. Biomed. Anal. 2004, 35, 259–266.
- Caccamese, S.; Caruso, C.; Parrinello, N.; Savarino, A. High-Performance Liquid Chromatographic Separation and Chiroptical Properties of the Enantiomers of Naringenin and Other Flavanones. J. Chromatogr. A 2005, 1076, 155–162.
- Ali, I.; Naim, L.; Ghanem, A.; Aboul-Enein, H.Y. Chiral Separations of Piperidine-2,6-Dione Analogues on Chiralpak IA and Chiralpak IB Columns by Using HPLC. Talanta 2006, 69, 1013–1017.
- Gazić, I.; Bosak, A.; Šinko, G.; Vinković, V.; Kovarik, Z. Preparative HPLC Separation of Bambuterol Enantiomers and Stereoselective Inhibition of Human Cholinesterases. Anal. Bioanal. Chem. 2006, 385, 1513–1519.
- Sun, P.; Wang, C.; Armstrong, D.; Péter, A.; Forró, E. Separation of Enantiomers of β-Lactams by HPLC Using Cyclodextrin-Based Chiral Stationary Phases. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1847–1860.
- Sun, P.; Krishnan, A.; Yadav, A.; Singh, S.; MacDonnell, F.M.; Armstrong, D.W. Enantiomeric Separations of Ruthenium (II) Polypyridyl Complexes Using HPLC With Cyclofructan Chiral Stationary Phases. Inorg. Chem. 2007, 46, 10312–10320.
- Hoffmann, C.V.; Pell, R.; Lämmerhofer, M.; Lindner, W. Synergistic Effects on Enantioselectivity of Zwitterionic Chiral Stationary Phases for Separations of Chiral Acids, Bases, and Amino Acids by HPLC. Anal. Chem. 2008, 80, 8780–8789.
- Ali, I.; Gaitonde, V.D.; Aboul-Enein, H.Y.; Hussain, A. Chiral Separation of β-Adrenergic Blockers on CelluCoat Column by HPLC. Talanta 2009, 78, 458–463.
- Zhou, Y.; Li, L.; Lin, K.; Zhu, X.; Liu, W. Enantiomer Separation of Triazole Fungicides by High-Performance Liquid Chromatography. Chirality 2009, 21, 421–427.
- Ye, J.; Yu, W.; Chen, G.; Shen, Z.; Zeng, S. Enantiomeric Separation of 2-Arylpropionic Acid Nonsteroidal Anti-Inflammatory Drugs by HPLC with Hydroxypropyl-β-Cyclodextrin as Chiral Mobile Phase Additive. Biomed. Chromatogr. 2010, 24, 799–807.
- Ali, I.; Al-Othman, Z.A.; Hussain, A.; Saleem, K.; Aboul-Enein, H.Y. Chiral Separation of β-Adrenergic Blockers in Human Plasma by SPE-HPLC. Chromatographia 2011, 73, 251–256.
- Bi, W.; Tian, M.; Row, K.H. Chiral Separation and Determination of Ofloxacin Enantiomers by Ionic Liquid-Assisted Ligand-Exchange Chromatography. Analyst 2011, 136, 379–387.
- Li, X.; Liu, Y.; Hu, C.; Bai, L.; Gao, B.; Huang, K. Direct Optical Resolution of Chiral Pesticides by High Performance Liquid Chromatography. Chin. J. Chem. Eng. 2011, 19, 603–609.
- Jibuti, G.; Mskhiladze, A.; Takaishvili, N.; Karchkhadze, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. HPLC Separation of Dihydropyridine Derivatives Enantiomers with Emphasis on Elution Order Using Polysaccharide-Based Chiral Columns. J. Sep. Sci. 2012, 35, 2529–2537.
- Matarashvili, I.; Chankvetadze, L.; Fanali, S.; Farkas, T.; Chankvetadze, B. HPLC Separation of Enantiomers of Chiral Arylpropionic Acid Derivatives Using Polysaccharide-Based Chiral Columns and Normal-Phase Eluents with Emphasis on Elution Order. J. Sep. Sci. 2013, 36, 140–147.
- Padivitage, N.L.T.; Dodbiba, E.; Breitbach, Z.S.; Armstrong, D.W. Enantiomeric Separations of Illicit Drugs and Controlled Substances Using Cyclofructan-Based (LARIHC) and Cyclobond I 2000 RSP HPLC Chiral Stationary Phases. Drug Test. Anal. 2014, 6, 542–551.
- Shu, Y.; Breitbach, Z.S.; Dissanayake, M.K.; Perera, S.; Aslan, J.M.; Alatrash, N.; MacDonnell, F.M.; Armstrong, D.W. Enantiomeric Separations of Ruthenium (II) Polypyridyl Complexes Using HPLC With Cyclofructan Chiral Stationary Phases. Chirality 2015, 27, 64–70.
- Broughton, D.B.; Gerhold, C.G. Continuous Sorption Process Employing Fixed Bed of Sorbent and Moving Inlets and Outlets. U.S. Patent US2985589A, 23 May 1961.
- Nicoud, R.M. Chromatographic Processes; Cambridge University Press: Cambridge, UK, 2015.
- Negawa, M.; Shoji, F. Optical Resolution by Simulated Moving-Bed Adsorption Technology. J. Chromatogr. A 1992, 590, 113–117.
- Cavoy, E.; Deltent, M.F.; Lehoucq, S.; Miggiano, D. Laboratory-Developed Simulated Moving Bed for Chiral Drug Separations. J. Chromatogr. A 1997, 769, 49–57.
- Devant, R.M.; Jonas, R.; Schulte, M.; Keil, A.; Charton, F. Enantiomer Separation of a Novel Ca-Sensitizing Drug by Simulated Moving Bed (SMB)—Chromatography. J. Prakt. Chem. 1997, 339, 315–321.
- Francotte, E.R.; Richert, P. Applications of Simulated Moving-Bed Chromatography to the Separation of the Enantiomers of Chiral Drugs. J. Chromatogr. A 1997, 769, 101–107.
- Pais, L.S.; Loureiro, J.M.; Rodrigues, A.E. Separation of 1,1′-Bi-2-Naphthol Enantiomers by Continuous Chromatography in Simulated Moving Bed. Chem. Eng. Sci. 1997, 52, 245–257.
- Pais, L.S.; Loureiro, J.M.; Rodrigues, A.E. Modeling, Simulation and Operation of a Simulated Moving Bed for Continuous Chromatographic Separation of 1,1′-Bi-2-Naphthol Enantiomers. J. Chromatogr. A 1997, 769, 25–35.
- Francotte, E.; Richert, P.; Mazzotti, M.; Morbidelli, M. Simulated Moving Bed Chromatographic Resolution of a Chiral Antitussive. J. Chromatogr. A 1998, 796, 239–248.
- Heuer, C.; Küsters, E.; Plattner, T.; Seidel-Morgenstern, A. Design of the Simulated Moving Bed Process Based on Adsorption Isotherm Measurements Using a Perturbation Method. J. Chromatogr. A 1998, 827, 175–191.
- Pais, L.S.; Loureiro, J.M.; Rodrigues, A.E. Modeling Strategies for Enantiomers Separation by SMB Chromatography. AIChE J. 1998, 44, 561–569.
- Khattabi, S.; Cherrak, D.E.; Mihlbachler, K.; Guiochon, G. Enantioseparation of 1-Phenyl-1-Propanol by Simulated Moving Bed under Linear and Nonlinear Conditions. J. Chromatogr. A 2000, 893, 307–319.
- Huthmann, E.; Juza, M. Modification of a Commercial Chiral Stationary Phase: Influences on Enantiomer Separations Using Simulated Moving Bed Chromatography. J. Chromatogr. A 2001, 908, 185–200.
- Francotte, E.; Leutert, T.; Vecchia, L.L.; Ossola, F.; Richert, P.; Schmidt, A. Preparative Resolution of the Enantiomers of Tert-Leucine Derivatives by Simulated Moving Bed Chromatography. Chirality 2002, 14, 313–317.
- Lee, K.B.; Chin, C.Y.; Xie, Y.; Cox, G.B.; Wang, N.L. Standing-Wave Design of a Simulated Moving Bed under a Pressure Limit for Enantioseparation of Phenylpropanolamine. Ind. Eng. Chem. Res. 2005, 44, 3249–3267.
- Amanullah, M.; Grossmann, C.; Mazzotti, M.; Morari, M.; Morbidelli, M. Experimental Implementation of Automatic “cycle to Cycle” Control of a Chiral Simulated Moving Bed Separation. J. Chromatogr. A 2007, 1165, 100–108.
- Choi, Y.J.; Han, S.K.; Chung, S.T.; Row, K.H. Separation of Racemic Bupivacaine Using Simulated Moving Bed with Mathematical Model. Biotechnol. Bioprocess. Eng. 2007, 12, 625–633.
- Zhang, L.; Gedicke, K.; Kuznetsov, M.A.; Staroverov, S.M.; Seidel-Morgenstern, A. Application of an Eremomycin-Chiral Stationary Phase for the Separation of Dl-Methionine Using Simulated Moving Bed Technology. J. Chromatogr. A 2007, 1162, 90–96.
- Araújo, J.M.M.; Rodrigues, R.C.R.; Eusébio, M.F.J.; Mota, J.P.B. On-Line Enantiomeric Analysis Using High-Performance Liquid Chromatography in Chiral Separation by Simulated Moving Bed. J. Chromatogr. A 2008, 1189, 292–301.
- Zabka, M.; Minceva, M.; Gomes, P.S.; Rodrigues, A.E. Chiral Separation of R,S-α- Tetralol by Simulated Moving Bed. Sep. Sci. Technol. 2008, 43, 727–765.
- Acetti, D.; Langel, C.; Brenna, E.; Fuganti, C.; Mazzotti, M. Intermittent Simulated Moving Bed Chromatographic Separation of (RS,RS)-2-(2,4-Difluorophenyl)Butane-1,2,3-Triol. J. Chromatogr. A 2010, 1217, 2840–2846.
- Langel, C.; Grossmann, C.; Jermann, S.; Mazzotti, M.; Morari, M.; Morbidelli, M. Experimental Optimizing Control of the Simulated Moving Bed Separation of Tröger’s Base Enantiomers. Ind. Eng. Chem. Res. 2010, 49, 11996–12003.
- Lee, E.; Park, M.B.; Kim, J.M.; Kim, W.S.; Kim, I.H. Simulated Moving-Bed for Separation of Mandelic Acid Racemic Mixtures. Korean J. Chem. Eng. 2010, 27, 231–234.
- Katsuo, S.; Mazzotti, M. Intermittent Simulated Moving Bed Chromatography: 2. Separation of Tröger’s Base Enantiomers. J. Chromatogr. A 2010, 1217, 3067–3075.
- Katsuo, S.; Langel, C.; Sandré, A.L.; Mazzotti, M. Intermittent Simulated Moving Bed Chromatography: 3. Separation of Tröger’s Base Enantiomers under Nonlinear Conditions. J. Chromatogr. A 2011, 1218, 9345–9352.
- Ribeiro, A.E.; Gomes, P.A.; Pais, L.; Rodrigues, A.E. Chiral Separation of Flurbiprofen Enantiomers by Preparative and Simulated Moving Bed Chromatography. Chirality 2011, 23, 602–611.
- Ribeiro, A.E.; Gomes, P.S.; Pais, L.S.; Rodrigues, A.E. Separation Science and Technology Chiral Separation of Ketoprofen Enantiomers by Preparative and Simulated Moving Bed Chromatography Chiral Separation of Ketoprofen Enantiomers by Preparative and Simulated Moving Bed Chromatography. Sep. Sci. Technol. 2011, 4611, 1726–1739.
- Gong, R.; Lin, X.; Li, P.; Yu, J.; Rodrigues, A.E. Experiment and Modeling for the Separation of Guaifenesin Enantiomers Using Simulated Moving Bed and Varicol Units. J. Chromatogr. A 2014, 1363, 242–249.
- Cunha, F.C.; Secchi, A.R.; de Souza, M.B.; Barreto, A.G. Separation of Praziquantel Enantiomers Using Simulated Moving Bed Chromatographic Unit with Performance Designed for Semipreparative Applications. Chirality 2019, 31, 583–591.
- Blehaut, J.; Nicoud, R.M. Recent Aspects in Simulated Moving Bed. Analusis 1998, 26, 60–70.
- Cunha, F.C. Chromatographic Separation of Praziquantel Racemate Using Simulated Moving Bed: From Unit Design to Dynamic Studies with Online Measurements; Universidade Federal do Rio de Janeiro: Rio de Janeiro, Brazil, 2021.
