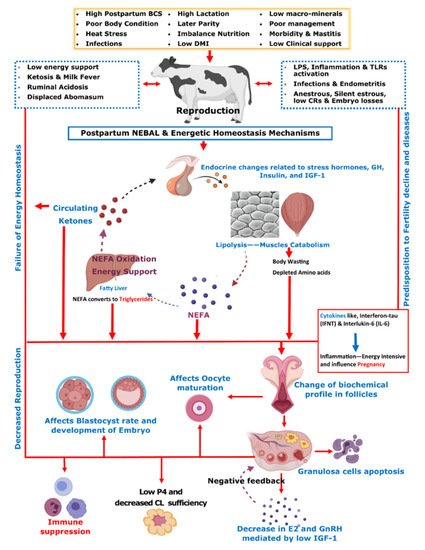
Initial post-parturient lactation is maintained at the expense of a decline in reproductive performance
[10]. The mechanism involving underlying NEBAL can be explained by low insulin levels causing an abundance of growth hormones (GHs), which in turn mobilize NEFAs. However, at the same time, there is a decrease in hepatic GH receptor abundance, preventing negative feedback through IGF-1
[38][49][38,49], while the presence of NEFAs is consistently behind the low insulin via catecholamines
[50]. As lipolysis helps the lactation demand, this is also the major cause of low BCS in postpartum cows. Thus, postpartum BCS is an indirect measurement of fat metabolism and correlates with the energy balance of cows
[51]. These aforementioned metabolic alterations mediated by complex endocrine changes have further consequences for ovulation, oocytes, and the corpus luteum
[38][49][52][53][54][38,49,52,53,54]. Further connections of these changes with reproductive performance include low concentrations of insulin and insulin growth factor 1 (IGF-1) causing follicular biochemical changes in ovaries and thus influencing luteinizing hormone (LH) and estradiol (E2) secretion
[52][53][55][52,53,55]. This decrease in LH and E2 secretions in turn ultimately leads to delayed resumption of the estrus cycle
[56]. On the other hand, NEBAL is also shown to be associated with low progesterone concentrations, which negatively influence the early pregnancy outcome
[57][58][57,58]. Carrying the discussion further, a high concentration of blood NEFAs is shown to be negatively associated with the developmental competence of fertilized oocytes
[59][60][59,60]. Generally, an increase above 0.55 mmol/L of plasma NEFA levels is regarded as a manifestation of serious postpartum NEBAL
[61][62][61,62]. Postpartum NEBAL and high NEFA concentrations have shown evidence for higher levels of inflammation characterized by cytokines and Toll-like receptor (TLR) expression leading to alterations in uterine functions
[63][64][65][63,64,65]. Furthermore, NEFA exerts cytotoxic effects at cellular levels and is attributed to immunosuppression
[66][67][66,67]. As NEBAL in itself possesses implications for early reproductive recovery, it is also obvious that postpartum NEBAL can be regarded as a root cause of various postpartum production diseases. Based on the discussions in this review,
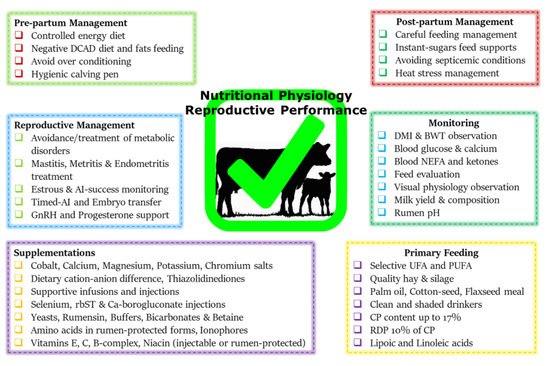
Figure 1 summarizes various metabolic and endocrine mechanisms contributing to the decline in post-parturient reproductive efficiency of dairy cows. A score of mitigation strategies have been advised to minimize extreme postpartum NEBAL incidence. Dry cow management should be oriented at the feeding of energy-rich diets over the duration of 3–4 weeks prepartum, in order to support fetal growth, the decline in DMI, and peri-parturient events
[68]. This phenomenon of increasing the energy content of the diet can also be supported by the facts of decreased DMI intake and rumination time during the last 3 weeks of gestation
[21][24][69][21,24,69]. However, over-conditioning of prepartum cows is not desirable and leads to postpartum metabolic disorders
[41][70][41,70]. A prepartum controlled energy diet near the calculated requirements essentially averted the risk of postpartum NEBAL
[70][71][70,71]. An increased energy prepartum diet is shown to enhance fat accumulation, decrease DMI, and increase the risk of health problems in postpartum dairy cows
[68][70][72][68,70,72]. Similarly, others have shown that an increased energy diet prepartum can lead to increased NEFA mobilization and triglyceride (TG) accumulation in the liver of postpartum cows
[73], while a controlled energy diet prepartum is shown to improve the immune function of postpartum dairy cows
[74]. Therefore, careful feeding management through monitoring of feed energy content and BCS assessment of prepartum cows constitute an essential key to perinatal transition success.