Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
Dendrimers (from the Greek dendros → tree; meros → part) are macromolecules with well-defined three-dimensional and tree-like structures. Remarkably, this hyperbranched architecture is one of the most ubiquitous, prolific, and recognizable natural patterns observed in nature.
- chromophoric dendrimers
1. Natural and Artificial Holistic-Integrated Molecular Systems
The rational design of methodological approaches in the quest forsatisfactory explanations of conceptual and empirical phenomena is the essence of holism [1][2]. In this context, it is highly relevant to understand the close relationship that exists in each of the parts that make up a given system and their interaction to form a whole [3]. Undoubtedly, this means that a whole is made up of parts, but the action that it exercises or performs cannot be unequivocally determined as the sum of its parts. This concept leads to thinking that the relationships and interactions between the parts contribute strongly to determining the qualities and attributes of the whole as a unit (i.e., the components are not independent of each other). In the historical context, the concept of holism in science was introduced in the second half of the 1920s, explaining the behavior of complex biological systems [4]. Considering this, in nature, it has been observed that the interaction among the component parts of a living organism (e.g., cells) acting as a whole allows for the preservation of their existence (Figure 1) [5][6][7].
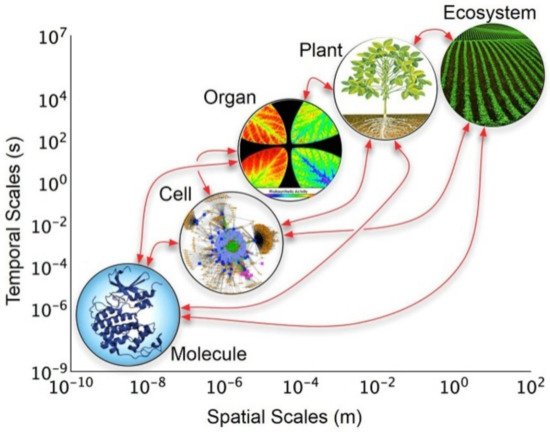
Figure 1. The holistic approach of hierarchical levels of organization of biological models across temporal and spatial scales. Reprinted with permission from reference [7]. Copyright 2017 Frontiers Media.
Therefore, the hierarchical interactions present in biological systems allow them to act as a whole, being more than the simple sum of their parts (i.e., hierarchical holism) [8][9]. Illustratively, inside the cell membrane, this behavior can also be observed because the coordinated interaction of their organelles (e.g., ribosomes, nucleus, mitochondrial) is fundamental for satisfying all needed biological functions (Figure 2) [10].
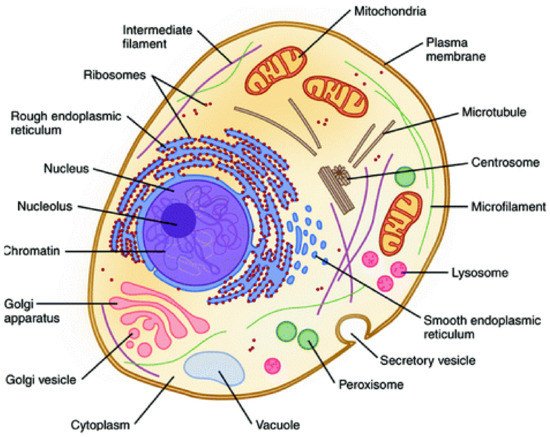
Figure 2. Representative structure of the cell showing their constituent organelles. Reprinted with permission from reference [10]. Copyright 2020 Springer.
A contrasting example is found in physics. The so-called entangled states belonging to quantum theory are often considered as an exception to the holistic principle. In this case, it is established that every system or physical entity can be described from its kinematic properties (e.g., velocity, position coordinates) in aform independent of other systems, i.e., a reductionist perspective. However, it is known that these properties can become dynamic (acting as more complex systems) when the influence of interactions with other systems is taken into account (Figure 3) [11]. Consequently, in the case of composting systems, the study of the state of an aggregate system is approached from the analysis of the states of the subsystems and their interactions.
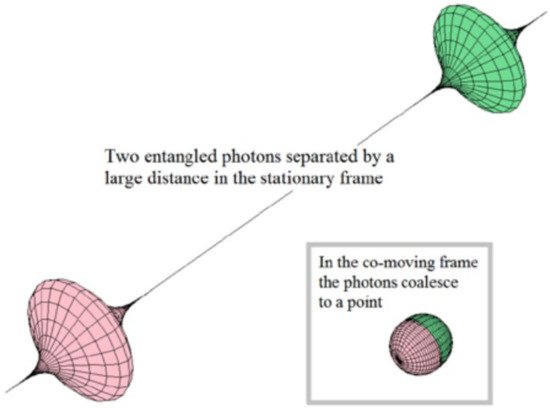
Figure 3. Illustration of two entangled photons. The state of one of them is instantaneously transferred to the other one. Reprinted with permission from [11]. Copyright 2015 Serials Publications.
On the other hand, the importance of integrated systems is closely related to the rapidly emerging field of nanoscience and nanotechnology [12][13]. Nanotechnology addresses the rational design and potential technological applications of functional structures that generally exhibit a high degree of organization at the molecular and supramolecular scale [14][15]. Importantly, an integrated molecular system can be considered as hierarchical assembled molecules or macromolecules arranged to carry out specific processes [16][17]. This is the case already described for biological systems (e.g., mitochondria, the photosynthetic system). Other comparatively less structurally complex but sophisticated artificial systems are abundantly reported in the literature, such as photoelectrochemical systems [18], sensors [19], semiconductors [20], and devices for solar cells [21], among others (Figure 4).
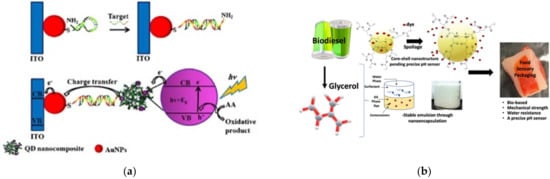
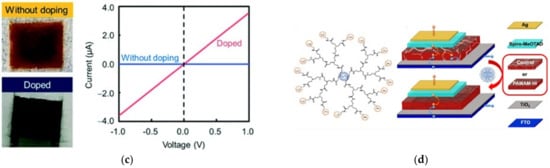
Figure 4. Dendrimer-based systems act as materials for (a) photocurrent generation, (b) sensor food spoilage detection, (c) semiconducting devices, and (d) enhancing the power conversion efficiency of solar cells. Reprinted with permission from [18] Copyright 2019 Springer; [19] Copyright 2021 American Chemical Society; [20] Copyright 2020 Royal Society of Chemistry; [21] Copyright 2021 Springer.
These are all representative examples of integrated molecular systems that constitute a considerable amount of knowledge and experience accumulated over several years towards the development of nanotechnology. For example, many efforts have been devoted to designing more thermodynamically efficient integrated photochemical systems, allowing the conversion of light energy into clean, abundant, and highly usable energy for technological purposes [22][23]. As mentioned above, this approach is based on mimicking, to some extent, the function and organization of biological systems on a scale of similar dimensions (e.g., micro, nanoscale). In general, these types of artificial systems have sophisticated structures and architectures, with a high degree of organization (i.e., assembled materials) [24]. From the perspective of technological advances, the fabrication of self-assembled structures (through intermolecular interactions) is an important drive towards designing photoresponsive systems [25]. This is a relevant point, since the challenge of mimicking more complex processes or structures that turn out to be more efficient is an interdisciplinary challenge fully in force to date [26][27]. Therefore, the scientific and technological endeavors should feel strongly encouraged to continue providing valuable efforts to better understand the role of functional holistic-integrated molecular systems [28][29].
2. Photo-Induced Energy Transfer Processes
Photo-induced energy transfer (PhET) involves light-induced processes present in molecular systems that contain photoactive chemical entities (e.g., chromophore groups) [30]. Globally, this type of process requires the study and an adequate understanding of the behavior of the excited states of organic molecules. Importantly, the thorough analysis of these processes can evidence valuable information on the light-induced energy-transfer mechanisms [31].
PhET processes are a highly relevant phenomenon both in nature and in artificial systems. A representative case is photosynthesis, wherein numerous energy transfer phenomena occur in the reaction center [32][33]. This triggers different mechanisms of ion separation and charges transport for the effective synthesis of energetic molecules. Thereby, photosynthesis can be visualized as large and complex machinery for converting light radiation into chemical energy [34]. In the case of artificial systems, photofunctional materials should exhibit attributes to harvest and convert solar energy into usable energy. Considering this, several examples can be cited as suitable molecular systems to favor photon-induced energy transfer, such as micelles [35], vesicles [36], colloids [37], monolayers [38], and dendrimers [39].
The above-mentioned systems require incorporating photoexcitable molecules or entities by hosting into their nanoreservoirs, intermolecular interactions, or forming covalent bonds. Ideally, photoexcitable molecules must contain active energy sites, donors, and acceptors. Additionally, the chemical environment would favor and stabilize the photoexcited species involved in the energy transfer process. Note that these phenomena occur at nanoscale dimensions; hence, the dimensions of the inner reservoirs of the host systems should be on that magnitude [40][41]. Considering these aspects, dendrimer-based systems can be conceived as a proper medium to ensure that the non-radiative process is raised for the lifetime of a fluorophore [42]. It is important to mention that the light-harvesting process performed by photoactive systems bearing electron-donor active sites involves the unidirectional transfer of the absorbed radiation energy [43]. This is an essential condition observed in nature, where photoactive centers composed of numerous chromophores exhibit a specific position or determine the spatial orientation for each other [44]. Therefore, artificial chemical systems would contain several symmetrically distributed light-harvesting donor–acceptor sites (into micellar, vesicular, or dendrimer structures) to appease a directional energy transfer over nanoscale dimensions. In recent years, the fluorescence (or Förster) resonance energy-transfer (FRET) mechanism has been studied in-depth to concentrate absorbed energy at a spatially confined site in nanometric regions [45][46][47]. FRET systems involve the presence of a significant amount of chromophores able to mainly absorb light radiation, e.g., in the visible region of the spectrum (this is addressed in more detail below). Importantly, these artificial systems would be suitable for generating multi-step mechanism directional energy transfer of the absorbed radiation energy [48]. These chemical environment conditions would allow for reaching photoactive centers separated by distances in the order of nanometers. Additionally, it is expected that the energy of photoexcitation of the chromophore centers corresponds typically to S0→S1 transitions (i.e., from HOMO to LUMO) [49][50].
2.1. FRET Phenomenon
This phenomenon, studied by Theodor Förster, consists of the transfer of light energy from an excited donor (D) unit to an acceptor (A) group through a non-radiative process (Figure 5) [51][52]. Importantly, this process is profited to estimate the separation distance at the molecular level between chromophores, such as that between a donor–acceptor pair. This is valid, mainly for separation distances smaller than 10 nm [53][54]. Thus, this phenomenon is highly sensitive to the spatial dimensions and specific orientation and the chemical nature of the environment surrounding the chromophore units. Thereby, FRET can be used as a reliable technique to characterize the dynamics, e.g.,the conformational changes of macromolecules, helping to establish the nature of intermolecular interactions in natural (e.g.,biological molecules) and artificial (e.g., micelles, dendrimers) nanosized systems having chromophores groups [38][55][56][57].
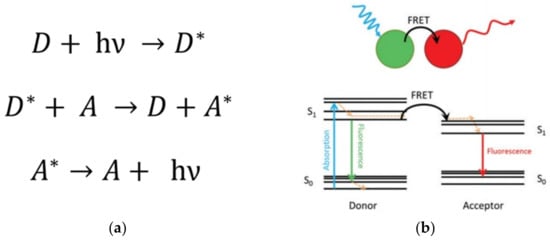
Figure 5. (a) Description of the FRET mechanism. D (A) and D* (A*) are the ground state and excited state of the donor unit (acceptor unit), respectively; h is the Planck’s constant; ν is the frequency of the radiation. (b) Representation of the FRET phenomenon by Jablonsky diagrams. The excited state of the donor unit (spatially close to the acceptor unit) reaches relaxation by the FRET mechanism exciting the acceptor (non-radiative energy transfer). The relaxation stage of the acceptor unit is evidenced by the fluorescence process emitting light. Note that the emitted light is a lower wavelength than the emission of the donor in a regular fluorescence process. Reprinted with permission from [52]. Copyright 2016 SAGE Publications.
References
- Seevinck, M.P. Holism, physical theories and quantum mechanics. Stud. Hist. Philos. Sci. Part B Stud. Hist. Philos. Mod. Phys. 2004, 35, 693–712.
- Weber, M.; Esfeld, M. Holism in the Sciences. In Unity of Knowledge (in Transdisciplinary Research for Sustainability); Encyclopedia of Life Support Systems Publishers: Abu Dhabi, United Arab Emirates, 2004.
- Banchetti-Robino, M.P. The Limits of Classical Extensional Mereology for the Formalization of Whole–Parts Relations in Quantum Chemical Systems. Philosophies 2020, 5, 16.
- Lawrence, C.; Weisz, G. Greater than the Parts: Holism in Biomedicine, 1920–1950; Oxford University Press: Oxford, UK, 1998.
- Shen, H.; Wang, Y.-Z.; Liu, G.; Li, L.; Xia, R.; Luo, B.; Wang, J.; Suo, D.; Shi, W.; Yong, Y.-C. A Whole-Cell Inorganic-Biohybrid System Integrated by Reduced Graphene Oxide for Boosting Solar Hydrogen Production. Am. Chem. Soc. Catal. 2020, 10, 13290–13295.
- He, Q.; Liao, Y.; Zhang, J.; Yao, X.; Zhou, W.; Hong, Y.; Ouyang, H. “All-in-One” Gel System for Whole Procedure of Stem-Cell Amplification and Tissue Engineering. Small 2020, 16, 1906539.
- Marshall-Colon, A.; Long, S.; Allen, D.; Allen, G.; Beard, D.; Benes, B.; von Caemmerer, S.; Christensen, A.; Cox, D.; Hart, J. Crops in silico: A prospectus from the Plants in silico symposium and workshop. Front. Plant Sci. 2017.
- Koestler, A. Beyond atomism and holism—The concept of the holon. Perspect. Biol. Med. 1970, 13, 131–154.
- Gupta, M.K.; Misra, K. A holistic approach for integration of biological systems and usage in drug discovery. Netw. Modeling Anal. Health Inform. Bioinform. 2016, 5, 4.
- Chappell, M.; Payne, S. Physiology for Engineers; Springer: Berlin/Heidelberg, Germany, 2020.
- Bergstrom, A. Apparent Superluminal Speeds in Evanescent Fields, Quantum Tunnelling and Quantum Entanglement. Int. J. Phys. 2015, 3, 40–44.
- Zhu, W.; Bartos, P.J.; Porro, A. Application of nanotechnology in construction. Mater. Struct. 2004, 37, 649–658.
- Lindsay, S. Introduction to Nanoscience; Oxford University Press: Oxford, UK, 2010.
- Ling, X.Y.; Reinhoudt, D.N.; Huskens, J. From supramolecular chemistry to nanotechnology: Assembly of 3D nanostructures. Pure Appl. Chem. 2009, 81, 2225–2233.
- Shrestha, L.K.; Mori, T.; Ariga, K. Dynamic nanoarchitectonics: Supramolecular polymorphism and differentiation, shape-shifter and hand-operating nanotechnology. Curr. Opin. Colloid Interface Sci. 2018, 35, 68–80.
- Jo, J.H.; Otanicar, T. A hierarchical methodology for the mesoscale assessment of building integrated roof solar energy systems. Renew. Energy 2011, 36, 2992–3000.
- Fei, Y.; Li, M.; Li, Y.; Wang, X.; Xue, C.; Wu, Z.; Xu, J.; Xiazeng, Z.; Cai, K.-Y.; Luo, Z. Hierarchical integration of degradable mesoporous silica nanoreservoirs and supramolecular dendrimer complex as a general-purpose tumor-targeted biomimetic nanoplatform for gene/small-molecule anticancer drug co-delivery. Nanoscale 2020, 12, 16102–16112.
- Divsar, F. A label-free photoelectrochemical DNA biosensor using a quantum dot–dendrimer nanocomposite. Anal. Bioanal. Chem. 2019, 411, 6867–6875.
- Pounds, K.; Jairam, S.; Bao, H.; Meng, S.; Zhang, L.; Godinez, S.; Savin, D.A.; Pelletier, W.; Correll, M.J.; Tong, Z. Glycerol-Based Dendrimer Nanocomposite Film as a Tunable pH-Sensor for Food Packaging. Am. Chem. Soc. Appl. Mater. Interfaces 2021, 13, 23268–23281.
- Oki, K.; Horike, S.; Yamaguchi, M.; Takechi, C.; Koshiba, Y.; Fukushima, T.; Mori, A.; Ishida, K. Thermoelectric thiophene dendrimers with large Seebeck coefficients. Mol. Syst. Des. Eng. 2020, 5, 809–814.
- Fan, W.; Du, Y.; Zhou, J.; Ma, X.; Liu, Y.; Xu, S.; Cao, S. NH3+-Functionalized PAMAM Dendrimers Enhancing Power Conversion Efficiency and Stability of Perovskite Solar Cells. J. Electron. Mater. 2021, 50, 6414–6425.
- Kathan, M.; Hecht, S. Photoswitchable molecules as key ingredients to drive systems away from the global thermodynamic minimum. Chem. Soc. Rev. 2017, 46, 5536–5550.
- Zhang, Z.; Bai, L.; Li, Z.; Qu, Y.; Jing, L. Review of strategies for the fabrication of heterojunctional nanocomposites as efficient visible-light catalysts by modulating excited electrons with appropriate thermodynamic energy. J. Mater. Chem. A 2019, 7, 10879–10897.
- Huang, J.; Wang, X.; Hogan, N.L.; Wu, S.; Lu, P.; Fan, Z.; Dai, Y.; Zeng, B.; Starko-Bowes, R.; Jian, J. Nanoscale artificial plasmonic lattice in self-assembled vertically aligned nitride–metal hybrid metamaterials. Adv. Sci. 2018, 5, 1800416.
- Matsuura, K.; Fujita, S. A Photoresponsive Artificial Viral Capsid Self-Assembled from an Azobenzene-Containing β-Annulus Peptide. Int. J. Mol. Sci. 2021, 22, 4028.
- Chang, T.H.; Li, K.; Yang, H.; Chen, P.Y. Multifunctionality and Mechanical Actuation of 2D Materials for Skin-Mimicking Capabilities. Adv. Mater. 2018, 30, 1802418.
- He, F.; Jeon, W.; Choi, W. Photocatalytic air purification mimicking the self-cleaning process of the atmosphere. Nat. Commun. 2021, 12, 2528.
- Gnansounou, E.; Alves, C.M. Integrated Sustainability Assessment of Biofuels. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–214.
- Abdullah, M.M.; Assi, A.T.; Asadalla, N.B. Integrated Ecosystem Sustainability Approach: Toward a Holistic System of Thinking of Managing Arid Ecosystems. Open J. Ecol. 2019, 9, 493.
- Alfonso-Hernandez, L.; Oldani, N.; Athanasopoulos, S.; Lupton, J.; Tretiak, S.; Fernandez-Alberti, S. Photoinduced Energy Transfer in Linear Guest–Host Chromophores: A Computational Study. J. Phys. Chem. A 2021, 125, 5303–5313.
- Puntoriero, F.; Nastasi, F.; Campagna, S.; Bura, T.; Ziessel, R. Vectorial photoinduced energy transfer between boron–dipyrromethene (Bodipy) chromophores across a fluorene bridge. Chem. Eur. J. 2010, 16, 8832–8845.
- Wasielewski, M.R. Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 1992, 92, 435–461.
- Sessler, J.L.; Wang, B.; Harriman, A. Photoinduced energy transfer in associated, but noncovalently-linked photosynthetic model systems. J. Am. Chem. Soc. 1995, 117, 704–714.
- Goudriaan, J.; Van Laar, H.; Van Keulen, H.; Louwerse, W. Photosynthesis, CO2 and plant production. In Wheat Growth and Modelling; Springer: Berlin/Heidelberg, Germany, 1985; pp. 107–122.
- Woo, H.Y.; Korystov, D.; Mikhailovsky, A.; Nguyen, T.-Q.; Bazan, G.C. Two-photon absorption in aqueous micellar solutions. J. Am. Chem. Soc. 2005, 127, 13794–13795.
- Nag, O.K.; Lim, C.S.; Nguyen, B.L.; Kim, B.; Jang, J.; Han, J.H.; Cho, B.R.; Woo, H.Y. pH-responsive water soluble smart vesicles containing a bis (styryl) benzene derivative for two-photon microscopy imaging. J. Mater. Chem. 2012, 22, 1977–1984.
- Strandwitz, N.C.; Khan, A.; Boettcher, S.W.; Mikhailovsky, A.A.; Hawker, C.J.; Nguyen, T.-Q.; Stucky, G.D. One-and two-photon induced polymerization of methylmethacrylate using colloidal CdS semiconductor quantum dots. J. Am. Chem. Soc. 2008, 130, 8280–8288.
- Peulen, T.-O.; Opanasyuk, O.; Seidel, C.A. Combining graphical and analytical methods with molecular simulations to analyze time-resolved FRET measurements of labeled macromolecules accurately. J. Phys. Chem. B 2017, 121, 8211–8241.
- Xu, B.; Zhang, J.; Fang, H.; Ma, S.; Chen, Q.; Sun, H.; Im, C.; Tian, W. Aggregation induced enhanced emission of conjugated dendrimers with a large intrinsic two-photon absorption cross-section. Polym. Chem. 2014, 5, 479–488.
- Sakamoto, A.; Mori, K.; Imura, K.; Okamoto, H. Nanoscale Two-Photon Induced Polymerization of Diacetylene Langmuir−Blodgett Film by Near-Field Photoirradiation. J. Phys. Chem. C 2011, 115, 6190–6194.
- Wu, S.; Ho, W. Two-photon-induced hot-electron transfer to a single molecule in a scanning tunneling microscope. Phys. Rev. B 2010, 82, 085444.
- Tian, W.; Yi, C.; Song, B.; Qi, Q.; Jiang, W.; Zheng, Y.; Qi, Z.; Sun, Y. Self-host homoleptic green iridium dendrimers based on diphenylamine dendrons for highly efficient single-layer PhOLEDs. J. Mater. Chem. C 2014, 2, 1104–1115.
- Zhang, Y.; Pei, L.; Zheng, Z.; Yuan, Y.; Xie, T.; Yang, J.; Chen, S.; Wang, J.; Waclawik, E.R.; Zhu, H. Heterojunctions between amorphous and crystalline niobium oxide with enhanced photoactivity for selective aerobic oxidation of benzylamine to imine under visible light. J. Mater. Chem. A 2015, 3, 18045–18052.
- Trost, J.T.; Blankenship, R.E. Isolation of a photoactive photosynthetic reaction center-core antenna complex from Heliobacillus mobilis. Biochemistry 1989, 28, 9898–9904.
- Imani, M.; Mohajeri, N.; Rastegar, M.; Zarghami, N. Recent advances in FRET-Based biosensors for biomedical applications. Anal. Biochem. 2021, 630, 114323.
- Jiang, N.; Wang, R.; You, X.; Geng, Y.; Zhu, D.; Zhang, N.; Bryce, M.R. Supramolecular oligourethane gels as light-harvesting antennae: Achieving multicolour luminescence and white-light emission through FRET. J. Mater. Chem. C 2021, 9, 13331–13337.
- Asher, W.B.; Geggier, P.; Holsey, M.D.; Gilmore, G.T.; Pati, A.K.; Meszaros, J.; Terry, D.S.; Mathiasen, S.; Kaliszewski, M.J.; McCauley, M.D. Single-molecule FRET imaging of GPCR dimers in living cells. Nat. Methods 2021, 18, 397–405.
- Gorbenko, G.; Zhytniakivska, O.; Vus, K.; Tarabara, U.; Trusova, V. Three-step Förster resonance energy transfer on amyloid fibril scaffold. Phys. Chem. Chem. Phys. 2021, 23, 14746–14754.
- Gong, Y.; Cole, J.M.; Ozoe, H.; Kawase, T. Photoexcited phenyl ring twisting in quinodimethane dyes enhances photovoltaic performance in dye-sensitized solar cells. Am. Chem. Soc. Appl. Energy Mater. 2018, 1, 1127–1139.
- Ghuman, K.K.; Hoch, L.B.; Szymanski, P.; Loh, J.Y.; Kherani, N.P.; El-Sayed, M.A.; Ozin, G.A.; Singh, C.V. Photoexcited surface frustrated Lewis pairs for heterogeneous photocatalytic CO2 reduction. J. Am. Chem. Soc. 2016, 138, 1206–1214.
- Hussain, S.A.; Dey, D.; Chakraborty, S.; Saha, J.; Roy, A.D.; Chakraborty, S.; Debnath, P.; Bhattacharjee, D. Fluorescence resonance energy transfer (FRET) sensor. Sci. Lett. J. 2015, 4, 119.
- Chamorro-Garcia, A.; Merkoçi, A. Nanobiosensors in diagnostics. Nanobiomedicine 2016, 3, 1849543516663574.
- Perrin, J. Fluorescence and molecular induction by resonance. CR Acad. Sci. Paris 1927, 184, 1097–1100.
- Clegg, R.M. The history of FRET. In Reviews in Fluorescence 2006; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–45.
- Keyvan Rad, J.; Mahdavian, A.R.; Salehi-Mobarakeh, H.; Abdollahi, A. FRET phenomenon in photoreversible dual-color fluorescent polymeric nanoparticles based on azocarbazole/spiropyran derivatives. Macromolecules 2016, 49, 141–152.
- Rashatasakhon, P.; Vongnam, K.; Siripornnoppakhun, W.; Vilaivan, T.; Sukwattanasinitt, M. FRET detection of DNA sequence via electrostatic interaction of polycationic phenyleneethynylene dendrimer with DNA/PNA hybrid. Talanta 2012, 88, 593–598.
- Morton, S.W.; Zhao, X.; Quadir, M.A.; Hammond, P.T. FRET-enabled biological characterization of polymeric micelles. Biomaterials 2014, 35, 3489–3496.
More
