Mycotoxins are toxic secondary metabolites produced by filamentous fungi in crops or during storage, transport and processing of food and feed commodities, which pose serious health risks for both humans and animals. The trend of mycotoxin contamination in food and feed has reached alarming levels. Antimicrobial peptides and proteins (AMPs) with antifungal activity are gaining much interest as natural antifungal compounds due to their properties such as structure diversity and function, antifungal spectrum, mechanism of action, high stability and the availability of biotechnological production methods.
- antimicrobial peptide (AMP)
- antifungal AMP
1. Introduction
The global challenge to prevent fungal spoilage and mycotoxin contamination on food and feed requires the development of new antifungal strategies. Given their multistep mode of action, the development of fungal resistance to AMPs is presumed to be slow or delayed compared to conventional fungicides. Interestingly, AMPs also accomplish important biological functions other than antifungal activity, including anti-mycotoxin biosynthesis activity, which opens novel aspects for their future use in agriculture and food industry to fight mycotoxin contamination. AMPs can reach intracellular targets and exert their activity by mechanisms other than membrane permeabilization. The mechanisms through which AMPs affect mycotoxin production are varied and complex, ranging from oxidative stress to specific inhibition of enzymatic components of mycotoxin biosynthetic pathways.
2. General Properties and Characteristics of Antimicrobial Peptides and Proteins (AMPs)
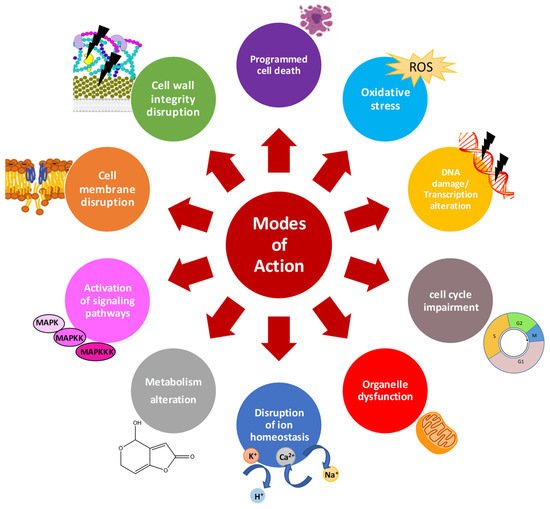
3. Effects of Distinct AMPs on Growth of Mycotoxin-Producing Fungi
3.1. Antifungal AMPs from Microorganisms
| Origin | Peptide | Target Fungi | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Bacillus amyloliquefaciens | Flagellin | F. oxysporum, A. niger | [85][44] | ||||
| B. subtilis | Fengycins | F. oxysporum | Cecropin-derived | Aspergillus spp., Fusarium spp.[86][45] | |||
| [ | 157 | , | 178][117][140] | B. subtilis | Iturin A | Aspergillus spp., Fusarium spp., Penicilium spp. | [87][46] |
| [ | 77 | ] | |||||
| γ-core | DefMT3, DefMT6, DefMT7-derived | F. graminearum, F. culmorum | [162,172][122][132] | B. thuringiensis | YvgO | B. fulva | [77][35 |
| MtDef4 | ] | ||||||
| M. truncatula | F. graminearum | ||||||
| K18M | Thanatin (8–21)-derived | [ | F. culmorum118][78] | [179][141] | Burkholderia cepacia | Cepacidines | A. niger |
| LfcinB17-31/LfcinB20-25 | Lactoferricin-derived | A. nidulans, F. oxysporum, P. expansum, Alternaria spp. | [88][47] | ||||
| [ | 175 | ] | [136] | Enterococcus durans | Duracin | F. culmorum | [89][48] |
| Lactic acid bacteria | Bacteriocins | A. parasiticus, P. expansum | [90,91][49][50] | ||||
| Lactobacillus brevis AM7 | Peptides | ||||||
| Pg-AMP1 | |||||||
| P. edulis | |||||||
| F. oxysporum | |||||||
| [ | |||||||
| 148 | |||||||
| ] | |||||||
| [ | |||||||
| 108 | |||||||
| ] | |||||||
3.3. Antifungal AMPs from Animal Origin
| Origin | Peptide | Target Fungi | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Invertebrate | ||||||||||
| Acanthoscurria gomesiana | Gomesin | Fusarium spp. | [156][116] | |||||||
| NaD1, NaD2 | ||||||||||
| P. roqueforti | ||||||||||
| Bombyx mori | Cecropin A | Aspergillus spp., Fusarium spp. | [157][117] | Nicotiana alata | ||||||
| Centruroides sculpturatus | BmKbpp2 | F. culmorum | [158][118] | |||||||
| MsrA1 | [ | 92 | ][51 | |||||||
| Drosophila melanogaster | Drosomycin | Fusarium spp., Aspergillus spp. | [159][119]] | |||||||
| F. graminearum | ||||||||||
| D. melanogaster | Metchnikowin | , F. oxysporum | [119][ | F. graminearum79] | ||||||
| [ | 160 | ] | [120] | OefDef1.1 | Olea europea | Cecropin: Melittin -derivedFusarium spp. | F. solani[120] | [180][142] | ||
| BP22 | de novo | P. expansum | [181][143] | L. paracasei | Bacteriocin F1 | P. glaucum, A. niger, A. flavus | [93][52] | |||
| L. plantarum | LR/14 | A. niger, P. chrysogenum | [94][53] | |||||||
| L. plantarum | FPSHTGMSVPPP | |||||||||
| Heliothis virescens | Heliomicin | Fusarium spp. | [80] | |||||||
| [ | 161 | ] | [121] | PvD1 | Phaseolus vulgaris | F. solani, F. oxysporum | ||||
| Ixodes ricinus | DefMT3, DefMT5, DefMT6 | F. graminearum, F. culmorum | [121][81] | |||||||
| [ | 162 | ] | [122] | Rs-AFP2 | Raphanus sativus | A. flavus, F. solani | [122][82] | |||
| Opistophtalmus carinatus | Opistoporin-1 | F. culmorum | [163][123] | TPP3 | ||||||
| Penaeid shrimps | N. tabacum | Penaeidins | Fusarium spp. | Aspergillus spp., F. oxysporum | [122][82] | |||||
| [ | 164 | ] | [124] | Hevein-type | ||||||
| Podisus maculiventris | Thanatin | A. brassicicola, F. culmorum | [150][110] | Aspergillus spp., P. roqueforti | [95][54] | |||||
| Ee-CBP | Euonymus europaeus | F. culmorum | [ | |||||||
| Pseudacanthotermes spiniger | 123 | ] | Termicin/Spinigerin[83] | |||||||
| Aspergillus | spp., | F. culmorum, F. oxysporym | [165][125] | L. plantarum TE10 | Peptides MIX | A. flavus | [ | GAFP78 | Ginkgo bilolba] | F. graminearum[36] |
| [ | 124 | ] | [84] | |||||||
| Sphodromantis viridis | Mastoparan-S | F. culmorum, A. niger, A. fumigatus | [166][126 | Streptomyces spp. | C/33-6 | F. graminearum | [96] | SmAMP3[55] | ||
| Stellariamedia | F. solani | [ | 125][85] | |||||||
| ] | ||||||||||
| Fish and Amphibians | S. tendae | Nikkomycin Z | Aspergillus spp., Fusarium spp., Penicilium spp. | [97][56] | ||||||
| Vaccatides | Vaccaria hispanica | Fusarium spp. | [126][86] | |||||||
| Phyllomedusa bicolor | Skin-PYY | A. niger | ||||||||
| D-V13K | de novo | Aspergillus spp. | [182][144[167][127] | S. tendae Tu901 | AFP1 | A. fumigatus | [98][57] | |||
| WAMP-1a and b | Triticum aestivum | F. moniliforme | ||||||||
| ] | Pleuronectes americanus | Pleurocidin | F. oxysporum, A. niger, Alternaria spp.[127][87] | |||||||
| [ | 151 | ] | [111] | Fungi | ||||||
| Napin | Aspergillus giganteous | AFP | Fusarium spp. | |||||||
| BoNap | Brassica oleracea | F. culmorum, P. expansum | [99][58] | |||||||
| [ | 128 | ] | [88] | A. clavatus | AcAFP | F. oxysporum, F. solani | [ | |||
| Snakins | 100 | ] | [59] | |||||||
| A. clavatus | AcAMP | F. oxysporum, F. solani | [101 | |||||||
| Snakin Z | Jujube fruits | ] | [ | A. niger60] | ||||||
| [ | 129 | ] | [89] | A. niger | Anafp | A. flavus, F. oxysporum, F. solani | [65][23] | |||
| Fusarium graminearum | FgAFP | F. verticilloides, F. proliferatum | [102][61] | |||||||
| Emericellopsis alkalina | Emericellipsin A | A.niger, A. flavus | [103][62] | |||||||
| Monascus pilosus | MAFP1 | Fusarium spp. | [104] | |||||||
| SN1, SN2 | Solanum tuberosum | [ | 63] | |||||||
| F. solani | , | F. culmorum | [ | Neosartoria fischeri | NFAP | A. nidulans, F. graminearum | [105][64] | |||
| N. fischeri | NFAP2 | A. nidulans | [106][65] | |||||||
| Penicillium citrinum | PcPAF | F. oxysporum | [107][66] | |||||||
| P. chrysogenum | PAF | F. oxysporum, A. flavus | [108][67] | |||||||
| P. chrysogenum | PgAFP/PAFB | F. oxysporum, A. flavus | [81,109][40][68] | |||||||
| P. chrysogenum | Pc-Arctin/PAFC | A. longipes, B. spectabilis | [66,110][24][69] | |||||||
| P. digitatum | PdAfpB | F. oxysporum, P. expansum | [82,111][41][70] | |||||||
| P. expansum | PeAfpA | A. alternata, Aspergillus spp., Byssochlamys spp., Fusarium spp., Penicillium spp. |
[82][41] | |||||||
| P. expansum | PeAfpB | Alternaria spp., Aspergillus spp., Byssochlamys spp., Fusarium spp., Penicillium spp. |
[82][41] | |||||||
| P. expansum | PeAfpC | A. flavus, Byssochlamys spp. | [82][41] |
3.2. Antifungal AMPs from Plants
| Peptide | Origin | Target Fungi | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Defensins | |||||||
| Ace-AMP1 | Allium cepa | F. solani, F. oxysporum | [115][75] | ||||
| Dm-AMP1 | Dahlia merkii | Fusarium spp. | [116][76] | ||||
| MsDef1 | Medicago sativa | F. graminearum | [117] | ||||
| (KW)n/(RW)n | |||||||
| de novo | |||||||
| F. solani | , | F. oxysporum | [183][145] | ||||
| O3TR/C12O3TR | de novo | F. culmorum, P. expansum, A. niger | [184][146] | ||||
| PAF26/PAF32 | de novo | Penicillium spp., F. oxysporum, | [75,175][33][136] | ||||
| PAF76/PAF77 | de novo | F. oxysporum | [185][147] | ||||
| PEP 6 | de novo | F. oxysporum | [185][147] | ||||
| PPD1/66-10/77-3 | de novo | A. flavus, A. parasiticus | [186][148] | ||||
| Mammals | |||||||
| Bovine | Cathelicidin BMAP-28 | Aspergillus spp., Penicillium spp. | [168][128] | ||||
| 130 | |||||||
| ] | |||||||
| Bovine | Indolicidin | [ | 90] | ||||
| A. niger | , | Penicillium spp. | [169][129] | ||||
| Bovine | Lactoferrin | A. niger | StSN1-2 | S. tuberosum | Fusarium spp., A. flavus | [131][91] | |
| Thaumatin-like | |||||||
| Osmotin | N. tabacum | F. solani, F. oxysporum | [98][57] | ||||
| Zeamatin | Zea mays | F. solani | [132][92] | ||||
| Thionins | |||||||
| Pth-St1 | S. tuberosum | F. solani | [133][93] | ||||
| Thionin 2.4 | Arabidopsis thaliana | F. graminearum | [134][94] | ||||
| [ | 153 | ] | [113] | ||||
| Human | Defensin HBD-3 | F. culmorum, P. expansum, A. niger. | [152][112] | ||||
| Human | Hepc20/Hepc25 | A. niger | [170][130] | Tu-AMP1, AMP2 | Tulipa gesneriana | F. oxysporum | [135][95] |
| Viscotoxin A3 | Viscum album | F. solani | [136][96] | ||||
| 2S albumin | |||||||
| Human | Tritrptcin | A. flavus | [171][131] | Bn-2S | Brassica napus | F. culmorum, F. oxysporum | [137][97] |
| CW-1 | Malva parviflora | F. graminearum | [138][98] | ||||
| Pe AFP1 | Passiflora edulis | F. oxysporum | [139][99] | ||||
| Pf2 | P. edulis | F. oxysporum | [140][100] | ||||
| LTPs | |||||||
| Bc-nsLTP | B. campestris | F. oxysporum | [141][101] | ||||
| Ca-LTp1 | Capsicum annuum | F. oxysporum | [142][102] | ||||
| Ha-AP10 | Helianthus annus | F. solani | [143][103] | ||||
| Knottins | |||||||
| Mj AMP2 | Mirabilis jalapa | F. oxysporum | [144][104] | ||||
| PAFP-s | Phytolacca american | F. oxysporum, F. graminearum | |||||
| Hairpinins | |||||||
| Sm-AMP-x2 | Stellaria media | F.oxysporum, A. niger, A. alternata | [145][105] | ||||
| Puroindolines | |||||||
| PIN-A | T. aestivum | F. culmorum | [146][106] | ||||
| PIN-B | Hordeum vulgare | F. graminearum | |||||
| Gly-rich peptides | |||||||
| Gc-GRP | Coffea canephora | F. oxysporum | [147][107] |
3.4. Synthetic Antifungal Peptides
References
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 453.
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230.
- Thery, T.; Lynch, K.M.; Arendt, E.K. Natural Antifungal Peptides/Proteins as Model for Novel Food Preservatives. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1327–1360.
- Van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and Mechanisms of Action of Naturally Occurring Antifungal Peptides. Cell. Mol. Life Sci. 2013, 70, 3545–3570.
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093.
- Do Nascimento Dias, J.; de Souza Silva, C.; de Araújo, A.R.; Souza, J.M.T.; de Holanda Veloso Júnior, P.H.; Cabral, W.F.; da Glória da Silva, M.; Eaton, P.; de Souza de Almeida Leite, J.R.; Nicola, A.M.; et al. Mechanisms of Action of Antimicrobial Peptides ToAP2 and NDBP-5.7 against Candida albicans Planktonic and Biofilm Cells. Sci. Rep. 2020, 10, 10327.
- Parvy, J.-P.; Yu, Y.; Dostalova, A.; Kondo, S.; Kurjan, A.; Bulet, P.; Lemaître, B.; Vidal, M.; Cordero, J.B. The Antimicrobial Peptide Defensin Cooperates with Tumour Necrosis Factor to Drive Tumour Cell Death in Drosophila. eLife 2019, 8, e45061.
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20.
- Struyfs, C.; Cools, T.L.; De Cremer, K.; Sampaio-Marques, B.; Ludovico, P.; Wasko, B.M.; Kaeberlein, M.; Cammue, B.P.A.; Thevissen, K. The Antifungal Plant Defensin HsAFP1 Induces Autophagy, Vacuolar Dysfunction and Cell Cycle Impairment in Yeast. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183255.
- Finking, R.; Marahiel, M.A. Biosynthesis of Nonribosomal Peptides. Annu. Rev. Microbiol. 2004, 58, 453–488.
- Wang, G. Post-Translational Modifications of Natural Antimicrobial Peptides and Strategies for Peptide Engineering. Curr. Biotechnol. 2012, 1, 72–79.
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250.
- Marcos, J.F.; Gandía, M. Antimicrobial Peptides: To Membranes and Beyond. Expert Opin. Drug Discov. 2009, 4, 659–671.
- Nicolas, P. Multifunctional Host Defense Peptides: Intracellular-Targeting Antimicrobial Peptides. FEBS J. 2009, 276, 6483–6496.
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal Peptides: To Be or Not to Be Membrane Active. Biochimie 2016, 130, 132–145.
- Nguyen, L.; Haney, E.; Vogel, H. The Expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472.
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395.
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.D.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic Development Challenges: The Various Mechanisms of Action of Antimicrobial Peptides and of Bacterial Resistance. Front. Microbiol. 2013, 4, 353.
- Hegedüs, N.; Marx, F. Antifungal Proteins: More than Antimicrobials? Fungal Biol. Rev. 2013, 26, 132–145.
- Sarkar, T.; Chetia, M.; Chatterjee, S. Antimicrobial Peptides and Proteins: From Nature’s Reservoir to the Laboratory and Beyond. Front. Chem. 2021, 9, 432.
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302.
- Jiao, R.; Cai, Y.; He, P.; Munir, S.; Li, X.; Wu, Y.; Wang, J.; Xia, M.; He, P.; Wang, G.; et al. Bacillus amyloliquefaciens YN201732 Produces Lipopeptides With Promising Biocontrol Activity Against Fungal Pathogen Erysiphe Cichoracearum. Front. Cell. Infect. Microbiol. 2021, 11, 387.
- Gun Lee, D.; Shin, S.Y.; Maeng, C.Y.; Jin, Z.Z.; Kim, K.L.; Hahm, K.S. Isolation and Characterization of a Novel Antifungal Peptide from Aspergillus niger. Biochem. Biophys. Res. Commun. 1999, 263, 646–651.
- Chen, Z.; Ao, J.; Yang, W.; Jiao, L.; Zheng, T.; Chen, X. Purification and Characterization of a Novel Antifungal Protein Secreted by Penicillium chrysogenum from an Arctic Sediment. Appl. Microbiol. Biotechnol. 2013, 97, 10381–10390.
- Moreno, A.B.; Martínez Del Pozo, A.; San Segundo, B. Biotechnologically Relevant Enzymes and Proteins. Antifungal Mechanism of the Aspergillus giganteus AFP against the Rice Blast Fungus Magnaporthe Grisea. Appl. Microbiol. Biotechnol. 2006, 72, 883–895.
- Silva, P.M.; Gonçalves, S.; Santos, N.C. Defensins: Antifungal Lessons from Eukaryotes. Front. Microbiol. 2014, 5, 97.
- Meyer, V.; Jung, S. Antifungal Peptides of the AFP Family Revisited: Are These Cannibal Toxins? Microorganisms 2018, 6, 50.
- Batta, G.; Barna, T.; Gáspári, Z.; Sándor, S.; Kövér, K.E.; Binder, U.; Sarg, B.; Kaiserer, L.; Chhillar, A.K.; Eigentler, A.; et al. Functional Aspects of the Solution Structure and Dynamics of PAF—A Highly-Stable Antifungal Protein from Penicillium chrysogenum. FEBS J. 2009, 276, 2875–2890.
- Campos-Olivas, R.; Bruix, M.; Santoro, J.; Lacadena, J.; Martinez del Pozo, A.; Gavilanes, J.G.; Rico, M. NMR Solution Structure of the Antifungal Protein from Aspergillus giganteus: Evidence for Cysteine Pairing Isomerism. Biochemistry 1995, 34, 3009–3021.
- Yount, N.Y.; Yeaman, M.R. Multidimensional Signatures in Antimicrobial Peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 7363–7368.
- Marcos, J.F.; Manzanares, P. Antimicrobial Peptides. In Antimicrobial Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 195–212. ISBN 978-0-470-59822-1.
- Kerenga, B.K.; McKenna, J.A.; Harvey, P.J.; Quimbar, P.; Garcia-Ceron, D.; Lay, F.T.; Phan, T.K.; Veneer, P.K.; Vasa, S.; Parisi, K.; et al. Salt-Tolerant Antifungal and Antibacterial Activities of the Corn Defensin ZmD32. Front. Microbiol. 2019, 10, 795.
- López-García, B.; Harries, E.; Carmona, L.; Campos-Soriano, L.; López, J.J.; Manzanares, P.; Gandía, M.; Coca, M.; Marcos, J.F. Concatemerization Increases the Inhibitory Activity of Short, Cell-Penetrating, Cationic and Tryptophan-Rich Antifungal Peptides. Appl. Microbiol. Biotechnol. 2015, 99, 8011–8021.
- Marcos, J.F.; Muñoz, A.; Pérez-Payá, E.; Misra, S.; López-García, B. Identification and Rational Design of Novel Antimicrobial Peptides for Plant Protection. Annu. Rev. Phytopathol. 2008, 46, 273–301.
- Manns, D.C.; Churey, J.J.; Worobo, R.W. Variable Efficacy of the Proteinaceous Antifungal YvgO in Select Fruit Juices and Teas as a Complement with UV Methods of Food Protection. J. Food Prot. 2015, 78, 1851–1860.
- Muhialdin, B.J.; Algboory, H.L.; Kadum, H.; Mohammed, N.K.; Saari, N.; Hassan, Z.; Meor Hussin, A.S. Antifungal Activity Determination for the Peptides Generated by Lactobacillus plantarum TE10 against Aspergillus flavus in Maize Seeds. Food Control. 2020, 109, 106898.
- Delgado, J.; Owens, R.A.; Doyle, S.; Asensio, M.A.; Núñez, F. Manuscript Title: Antifungal Proteins from Moulds: Analytical Tools and Potential Application to Dry-Ripened Foods. Appl. Microbiol. Biotechnol. 2016, 100, 6991–7000.
- Garrigues, S.; Gandía, M.; Marcos, J.F. Occurrence and Function of Fungal Antifungal Proteins: A Case Study of the Citrus Postharvest Pathogen Penicillium digitatum. Appl. Microbiol. Biotechnol. 2016, 100, 2243–2256.
- Leiter, É.; Gáll, T.; Csernoch, L.; Pócsi, I. Biofungicide Utilizations of Antifungal Proteins of Filamentous Ascomycetes: Current and Foreseeable Future Developments. BioControl 2017, 62, 125–138.
- Delgado, J.; Acosta, R.; Rodríguez-Martín, A.; Bermúdez, E.; Núñez, F.; Asensio, M.A. Growth Inhibition and Stability of PgAFP from Penicillium chrysogenum against Fungi Common on Dry-Ripened Meat Products. Int. J. Food Microbiol. 2015, 205, 23–29.
- Martínez-Culebras, P.V.; Gandía, M.; Boronat, A.; Marcos, J.F.; Manzanares, P. Differential Susceptibility of Mycotoxin-Producing Fungi to Distinct Antifungal Proteins (AFPs). Food Microbiol. 2021, 97, 103760.
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009; p. 519. ISBN 978-0-387-92206-5.
- Delgado, J.; Ballester, A.-R.; Núñez, F.; González-Candelas, L. Evaluation of the Activity of the Antifungal PgAFP Protein and Its Producer Mould against Penicillium spp. Postharvest Pathogens of Citrus and Pome Fruits. Food Microbiol. 2019, 84, 103266.
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a Potential Biocontrol Agent against Fusarium oxysporum f. sp. spinaciae. J. Basic Microbiol. 2014, 54, 448–456.
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901.
- Klich, M.; Lax, A.; Bland, J. Inhibition of Some Mycotoxigenic Fungi by Iturin A, a Peptidolipid Produced by Bacillus Subtilis. Mycopathologia 1991, 116, 77–80.
- Lim, Y.; Suh, J.W.; Kim, S.; Hyun, B.; Kim, C.; Lee, C.H. Cepacidine A, a Novel Antifungal Antibiotic Produced by Pseudomonas Cepacia. II. Physico-Chemical Properties and Structure Elucidation. J. Antibiot. 1994, 47, 1406–1416.
- Belguesmia, Y.; Choiset, Y.; Rabesona, H.; Baudy-Floc’h, M.; Le Blay, G.; Haertlé, T.; Chobert, J.-M. Antifungal Properties of Durancins Isolated from Enterococcus durans A5-11 and of Its Synthetic Fragments. Lett. Appl. Microbiol. 2012, 56.
- Daie Ghazvini, R.; Kouhsari, E.; Zibafar, E.; Hashemi, J.; Amini, A.; Niknejad, F. Antifungal Activity and Aflatoxin Degradation of Bifidobacterium Bifidum and Lactobacillus Fermentum Against Toxigenic Aspergillus parasiticus. Open Microbiol. J. 2016, 10, 1–5.
- Luz, C.; Saladino, F.; Luciano, F.B.; Mañes, J.; Meca, G. In Vitro Antifungal Activity of Bioactive Peptides Produced by Lactobacillus plantarum against Aspergillus parasiticus and Penicillium expansum. LWT—Food Sci. Technol. 2017, 81, 128–135.
- Coda, R.; Rizzello, C.G.; Nigro, F.; De Angelis, M.; Arnault, P.; Gobbetti, M. Long-Term Fungal Inhibitory Activity of Water-Soluble Extracts of Phaseolus Vulgaris Cv. Pinto and Sourdough Lactic Acid Bacteria during Bread Storage. Appl. Environ. Microbiol. 2008, 74, 7391–7398.
- Miao, J.; Guo, H.; Ou, Y.; Liu, G.; Fang, X.; Liao, Z.; Ke, C.; Chen, Y.; Zhao, L.; Cao, Y. Purification and Characterization of Bacteriocin F1, a Novel Bacteriocin Produced by Lactobacillus paracasei subsp. Tolerans FX-6 from Tibetan Kefir, a Traditional Fermented Milk from Tibet, China. Food Control. 2014, 42, 48–53.
- Gupta, R.; Srivastava, S. Antifungal Effect of Antimicrobial Peptides (AMPs LR14) Derived from Lactobacillus plantarum Strain LR/14 and Their Applications in Prevention of Grain Spoilage. Food Microbiol. 2014, 42, 1–7.
- Muhialdin, B.J.; Hassan, Z.; Bakar, F.A.; Saari, N. Identification of Antifungal Peptides Produced by Lactobacillus plantarum IS10 Grown in the MRS Broth. Food Control. 2016, 59, 27–30.
- Fulgueira, C.L.; Amigot, S.L.; Magni, C. Growth Inhibition of Toxigenic Fungi by a Proteinaceous Compound from Streptomyces sp. C/33-6. Curr. Microbiol. 2004, 48, 135–139.
- Li, R.K.; Rinaldi, M.G. In Vitro Antifungal Activity of Nikkomycin Z in Combination with Fluconazole or Itraconazole. Antimicrob. Agents Chemother. 1999, 43, 1401–1405.
- Freitas, C.; Nogueira, F.; Vasconcelos, I.; Oliveira, J.; Domont, G.; Ramos, M. Osmotin Purified from the Latex of Calotropis procera: Biochemical Characterization, Biological Activity and Role in Plant Defense. Plant. Physiol. Biochem. PPB/Soc. Fr. De Physiol. Veg. 2011, 49, 738–743.
- Theis, T.; Marx, F.; Salvenmoser, W.; Stahl, U.; Meyer, V. New Insights into the Target Site and Mode of Action of the Antifungal Protein of Aspergillus giganteus. Res. Microbiol. 2005, 156, 47–56.
- Skouri-Gargouri, H.; Gargouri, A. First Isolation of a Novel Thermostable Antifungal Peptide Secreted by Aspergillus clavatus. Peptides 2008, 29, 1871–1877.
- Mohamed, H.; Jellouli, K.; Hmidet, N.; Balti, R.; Sellami-Kamoun, A. A Highly Thermostable Antimicrobial Peptide from Aspergillus clavatus ES1: Biochemical and Molecular Characterization. J. Ind. Microbiol. Biotechnol. 2010, 37, 805–813.
- Patiño, B.; Vázquez, C.; Manning, J.M.; Roncero, M.I.G.; Córdoba-Cañero, D.; Di Pietro, A.; Martínez-del-Pozo, Á. Characterization of a Novel Cysteine-Rich Antifungal Protein from Fusarium graminearum with Activity against Maize Fungal Pathogens. Int. J. Food Microbiol. 2018, 283, 45–51.
- Rogozhin, E.A.; Sadykova, V.S.; Baranova, A.A.; Vasilchenko, A.S.; Lushpa, V.A.; Mineev, K.S.; Georgieva, M.L.; Kul’ko, A.B.; Krasheninnikov, M.E.; Lyundup, A.V.; et al. A Novel Lipopeptaibol Emericellipsin A with Antimicrobial and Antitumor Activity Produced by the Extremophilic Fungus Emericellopsis alkalina. Molecules 2018, 23, 2785.
- Tu, C.-Y.; Chen, Y.-P.; Yu, M.-C.; Hwang, I.-E.; Wu, D.-Y.; Liaw, L.-L. Characterization and Expression of the Antifungal Protein from Monascus pilosus and Its Distribution among Various Monascus Species. J. Biosci. Bioeng. 2016, 122, 27–33.
- Kovács, L.; Virágh, M.; Takó, M.; Papp, T.; Vágvölgyi, C.; Galgóczy, L. Isolation and Characterization of Neosartorya fischeri Antifungal Protein (NFAP). Peptides 2011, 32, 1724–1731.
- Tóth, L.; Kele, Z.; Borics, A.; Nagy, L.G.; Váradi, G.; Virágh, M.; Takó, M.; Vágvölgyi, C.; Galgóczy, L. NFAP2, a Novel Cysteine-Rich Anti-Yeast Protein from Neosartorya fischeri NRRL 181: Isolation and Characterization. AMB Express 2016, 6, 75.
- Wen, C.; Guo, W.; Chen, X. Purification and Identification of a Novel Antifungal Protein Secreted by Penicillium citrinum from the Southwest Indian Ocean. J. Microbiol. Biotechnol. 2014, 24, 1337–1345.
- Kaiserer, L.; Oberparleiter, C.; Weiler-Görz, R.; Burgstaller, W.; Leiter, E.; Marx, F. Characterization of the Penicillium chrysogenum Antifungal Protein PAF. Arch. Microbiol. 2003, 180, 204–210.
- Huber, A.; Hajdu, D.; Bratschun-Khan, D.; Gáspári, Z.; Varbanov, M.; Philippot, S.; Fizil, Á.; Czajlik, A.; Kele, Z.; Sonderegger, C.; et al. New Antimicrobial Potential and Structural Properties of PAFB: A Cationic, Cysteine-Rich Protein from Penicillium chrysogenum Q176. Sci. Rep. 2018, 8, 1751.
- Holzknecht, J.; Kühbacher, A.; Papp, C.; Farkas, A.; Váradi, G.; Marcos, J.F.; Manzanares, P.; Tóth, G.K.; Galgóczy, L.; Marx, F. The Penicillium chrysogenum Q176 Antimicrobial Protein PAFC Effectively Inhibits the Growth of the Opportunistic Human Pathogen Candida albicans. JoF 2020, 6, 141.
- Garrigues, S.; Gandía, M.; Popa, C.; Borics, A.; Marx, F.; Coca, M.; Marcos, J.F.; Manzanares, P. Efficient Production and Characterization of the Novel and Highly Active Antifungal Protein AfpB from Penicillium digitatum. Sci. Rep. 2017, 7, 14663.
- Gandía, M.; Monge, A.; Garrigues, S.; Orozco, H.; Giner-Llorca, M.; Marcos, J.F.; Manzanares, P. Novel Insights in the Production, Activity and Protective Effect of Penicillium expansum Antifungal Proteins. Int. J. Biol. Macromol. 2020, 164, 3922–3931.
- Marcos López, J.F.; Gandía Gómez, M.; Garrigues, S.; Manzanares, P.; Coca, M. Antifungal Peptides and Proteins with Activity against Fungi Causing Postharvest Decay; Taylor & Francis: New York, NY, USA, 2020; ISBN 978-1-315-20918-0.
- Yan, J.; Yuan, S.-S.; Jiang, L.-L.; Ye, X.-J.; Ng, T.; Wu, Z.-J. Plant Antifungal Proteins and Their Applications in Agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 4961–4981.
- Shwaiki, L.N.; Lynch, K.M.; Arendt, E.K. Future of Antimicrobial Peptides Derived from Plants in Food Application—A Focus on Synthetic Peptides. Trends Food Sci. Technol. 2021, 112, 312–324.
- Wu, Y.; He, Y.; Ge, X. Functional Characterization of the Recombinant Antimicrobial Peptide Trx-Ace-AMP1 and Its Application on the Control of Tomato Early Blight Disease. Appl. Microbiol. Biotechnol. 2011, 90, 1303–1310.
- Sagaram, U.S.; El-Mounadi, K.; Buchko, G.W.; Berg, H.R.; Kaur, J.; Pandurangi, R.S.; Smith, T.J.; Shah, D.M. Structural and Functional Studies of a Phosphatidic Acid-Binding Antifungal Plant Defensin MtDef4: Identification of an RGFRRR Motif Governing Fungal Cell Entry. PLoS ONE 2013, 8, e82485.
- Cruz, L.P.; Ribeiro, S.F.F.; Carvalho, A.O.; Vasconcelos, I.M.; Rodrigues, R.; Cunha, M.D.; Gomes, V.M. Isolation and Partial Characterization of a Novel Lipid Transfer Protein (LTP) and Antifungal Activity of Peptides from Chilli Pepper Seeds. Protein Pept. Lett. 2010, 17, 311–318.
- Kaur, J.; Thokala, M.; Robert-Seilaniantz, A.; Zhao, P.; Peyret, H.; Berg, H.; Pandey, S.; Jones, J.; Shah, D. Subcellular Targeting of an Evolutionarily Conserved Plant Defensin MtDef4.2 Determines the Outcome of Plant-Pathogen Interaction in Transgenic Arabidopsis. Mol. Plant. Pathol. 2012, 13, 1032–1046.
- Dracatos, P.M.; van der Weerden, N.L.; Carroll, K.T.; Johnson, E.D.; Plummer, K.M.; Anderson, M.A. Inhibition of Cereal Rust Fungi by Both Class I and II Defensins Derived from the Flowers of Nicotiana alata. Mol. Plant. Pathol. 2013, 15, 67–79.
- Li, H.; Velivelli, S.; Shah, D. Antifungal Potency and Modes of Action of a Novel Olive Tree Defensin Against Closely Related Ascomycete Fungal Pathogens. Mol. Plant.-Microbe Interact. 2019, 32, 1649–1664.
- Games, P.D.; Dos Santos, I.S.; Mello, E.O.; Diz, M.S.S.; Carvalho, A.O.; de Souza-Filho, G.A.; Da Cunha, M.; Vasconcelos, I.M.; Ferreira, B.D.S.; Gomes, V.M. Isolation, Characterization and Cloning of a CDNA Encoding a New Antifungal Defensin from Phaseolus vulgaris L. Seeds. Peptides 2008, 29, 2090–2100.
- Baxter, A.A.; Richter, V.; Lay, F.T.; Poon, I.K.H.; Adda, C.G.; Veneer, P.K.; Phan, T.K.; Bleackley, M.R.; Anderson, M.A.; Kvansakul, M.; et al. The Tomato Defensin TPP3 Binds Phosphatidylinositol (4,5)-Bisphosphate via a Conserved Dimeric Cationic Grip Conformation to Mediate Cell Lysis. Mol. Cell. Biol. 2015, 35, 1964–1978.
- Van den Bergh, K.P.B.; Proost, P.; Van Damme, J.; Coosemans, J.; Van Damme, E.J.M.; Peumans, W.J. Five Disulfide Bridges Stabilize a Hevein-Type Antimicrobial Peptide from the Bark of Spindle Tree (Euonymus europaeus L.). FEBS Lett. 2002, 530, 181–185.
- Wong, K.H.; Tan, W.L.; Kini, S.G.; Xiao, T.; Serra, A.; Sze, S.K.; Tam, J.P. Vaccatides: Antifungal Glutamine-Rich Hevein-Like Peptides from Vaccaria hispanica. Front. Plant. Sci. 2017, 8, 1100.
- Rogozhin, E.; Slezina, M.; Slavokhotova, A.; Istomina, E.; Korostyleva, T.; Smirnov, A.; Grishin, E.; Egorov, T.; Odintsova, T. A Novel Antifungal Peptide from Leaves of the Weed Stellaria media L. Biochimie 2015, 116, 125–132.
- Huang, R.-H.; Xiang, Y.; Liu, X.-Z.; Zhang, Y.; Hu, Z.; Wang, D.-C. Two Novel Antifungal Peptides Distinct with a Five-Disulfide Motif from the Bark of Eucommia ulmoides Oliv. FEBS Lett 2002, 521, 87–90.
- Odintsova, T.I.; Vassilevski, A.A.; Slavokhotova, A.A.; Musolyamov, A.K.; Finkina, E.I.; Khadeeva, N.V.; Rogozhin, E.A.; Korostyleva, T.V.; Pukhalsky, V.A.; Grishin, E.V.; et al. A Novel Antifungal Hevein-Type Peptide from Triticum kiharae Seeds with a Unique 10-Cysteine Motif. FEBS J. 2009, 276, 4266–4275.
- Thery, T.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Isolation, Characterisation and Application of a New Antifungal Protein from Broccoli Seeds—New Food Preservative with Great Potential. Food Control. 2020, 117, 107356.
- Daneshmand, F.; Zare-Zardini, H.; Ebrahimi, L. Investigation of the Antimicrobial Activities of Snakin-Z, a New Cationic Peptide Derived from Zizyphus jujuba Fruits. Nat. Prod. Res. 2013, 27, 2292–2296.
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and Antimicrobial Proteins and Peptides of Potato (Solanum tuberosum L.) Tubers and Their Applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547.
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; López, G.; García-Olmedo, F.; Molina, A. Snakin-2, an Antimicrobial Peptide from Potato Whose Gene Is Locally Induced by Wounding and Responds to Pathogen Infection. Plant. Physiol. 2002, 128, 951–961.
- Abad, L.R.; D’Urzo, M.P.; Liu, D.; Narasimhan, M.L.; Reuveni, M.; Zhu, J.K.; Niu, X.; Singh, N.K.; Hasegawa, P.M.; Bressan, R.A. Antifungal Activity of Tobacco Osmotin Has Specificity and Involves Plasma Membrane Permeabilization. Plant. Sci. 1996, 118, 11–23.
- Moreno, M.; Segura, A.; García-Olmedo, F. Pseudothionin-St1, a Potato Peptide Active against Potato Pathogens. Eur. J. Biochem. 1994, 223, 135–139.
- Asano, T.; Miwa, A.; Maeda, K.; Kimura, M.; Nishiuchi, T. The Secreted Antifungal Protein Thionin 2.4 in Arabidopsis thaliana Suppresses the Toxicity of a Fungal Fruit Body Lectin from Fusarium graminearum. PLOS Pathog. 2013, 9, e1003581.
- Fujimura, M.; Ideguchi, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, Characterization, and Sequencing of Novel Antimicrobial Peptides, Tu-AMP 1 and Tu-AMP 2, from Bulbs of Tulip (Tulipa esneriana L.). Biosci. Biotechnol. Biochem. 2004, 68, 571–577.
- Giudici, M.; Poveda, J.A.; Molina, M.L.; de la Canal, L.; González-Ros, J.M.; Pfüller, K.; Pfüller, U.; Villalaín, J. Antifungal Effects and Mechanism of Action of Viscotoxin A3. FEBS J. 2006, 273, 72–83.
- Ngai, P.H.K.; Ng, T.B. A Napin-like Polypeptide from Dwarf Chinese White Cabbage Seeds with Translation-Inhibitory, Trypsin-Inhibitory, and Antibacterial Activities. Peptides 2004, 25, 171–176.
- Wang, X.; Bunkers, G. Potent Heterologous Antifungal Proteins from Cheeseweed (Malva parviflora). Biochem. Biophys. Res. Commun. 2001, 279, 669–673.
- Pelegrini, P.; Noronha, E.; Muniz, M.A.R.; Vasconcelos, I.; CHIARELLO, M.; Oliveira, J.T.A.; Franco, O. An Antifungal Peptide from Passion Fruit (Passiflora edulis) Seeds with Similarities to 2S Albumin Proteins. Biochim. Et Biophys. Acta 2006, 1764, 1141–1146.
- Agizzio, A.P.; Carvalho, A.O.; Ribeiro, S.D.F.F.; Machado, O.L.T.; Alves, E.W.; Okorokov, L.A.; Samarão, S.S.; Bloch, C.; Prates, M.V.; Gomes, V.M. A 2S Albumin-Homologous Protein from Passion Fruit Seeds Inhibits the Fungal Growth and Acidification of the Medium by Fusarium oxysporum. Arch. Biochem. Biophys. 2003, 416, 188–195.
- Lin, P.; Xia, L.; Wong, J.H.; Ng, T.B.; Ye, X.; Wang, S.; Xiangzhu, S. Lipid Transfer Proteins from Brassica campestris and Mung Bean Surpass Mung Bean Chitinase in Exploitability. J. Pept. Sci. 2007, 13, 642–648.
- Diz, M.; de Oliveira Carvalho, A.; Ribeiro, S.; Cunha, M.; Beltramini, L.; Rodrigues, R.; Nascimento, V.; Machado, O.; Gomes, V. Characterisation, Immunolocalisation and Antifungal Activity of a Lipid Transfer Protein from Chili Pepper (Capsicum annuum) Seeds with Novel α-Amylase Inhibitory Properties. Physiol. Plant. 2011, 142, 233–246.
- Regente, M.; de la Canal, L. Purification, Characterization and Antifungal Properties of a Lipid-Transfer Protein from Sunflower (Helianthus annuus) Seeds. Physiol. Plant. 2000, 110, 158–163.
- Cammue, B.P.; De Bolle, M.F.; Terras, F.R.; Proost, P.; Van Damme, J.; Rees, S.B.; Vanderleyden, J.; Broekaert, W.F. Isolation and Characterization of a Novel Class of Plant Antimicrobial Peptides Form Mirabilis jalapa L. Seeds. J. Biol. Chem. 1992, 267, 2228–2233.
- Utkina, L.L.; Andreev, Y.A.; Rogozhin, E.A.; Korostyleva, T.V.; Slavokhotova, A.A.; Oparin, P.B.; Vassilevski, A.A.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. Genes Encoding 4-Cys Antimicrobial Peptides in Wheat Triticum kiharae Dorof. et Migush.: Multimodular Structural Organization, Instraspecific Variability, Distribution and Role in Defence. FEBS J. 2013, 280, 3594–3608.
- Charnet, P.; Molle, G.; Marion, D.; Rousset, M.; Lullien-Pellerin, V. Puroindolines Form Ion Channels in Biological Membranes. Biophys. J. 2003, 84, 2416–2426.
- Zottich, U.; Da Cunha, M.; Carvalho, A.O.; Dias, G.B.; Casarin, N.; Vasconcelos, I.M.; Gomes, V.M. An Antifungal Peptide from Coffea canephora Seeds with Sequence Homology to Glycine-Rich Proteins Exerts Membrane Permeabilization and Nuclear Localization in Fungi. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3509–3516.
- Pelegrini, P.B.; Murad, A.M.; Silva, L.P.; dos Santos, R.C.P.; Costa, F.T.; Tagliari, P.D.; Bloch, C., Jr.; Noronha, E.F.; Miller, R.N.G.; Franco, O.L. Identification of a Novel Storage Glycine-Rich Peptide from Guava (Psidium guajava) Seeds with Activity against Gram-Negative Bacteria. Peptides 2008, 29, 1271–1279.
- López-Meza, J.; Ochoa-Zarzosa, A.; Aguilar, J.; Loeza-Lara, P. Antimicrobial Peptides: Diversity and Perspectives for Their Biomedical Application. In Biomedical Engineering, Trends, Research and Technologies; IntechOpen: London, UK, 2011; ISBN 978-953-307-514-3.
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int. J. Mol. Sci. 2021, 22, 1522.
- Souza, A.L.A.; Díaz-Dellavalle, P.; Cabrera, A.; Larrañaga, P.; Dalla-Rizza, M.; De-Simone, S.G. Antimicrobial Activity of Pleurocidin Is Retained in Plc-2, a C-Terminal 12-Amino Acid Fragment. Peptides 2013, 45, 78–84.
- Thery, T.; Tharappel, J.C.; Kraszewska, J.; Beckett, M.; Bond, U.; Arendt, E.K. Antifungal Activity of a Synthetic Human β-Defensin 3 and Potential Applications in Cereal-Based Products. Innov. Food Sci. Emerg. Technol. 2016, 38, 160–168.
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2.
- Muñoz, A.; Marcos, J.F. Activity and Mode of Action against Fungal Phytopathogens of Bovine Lactoferricin-Derived Peptides. J. Appl. Microbiol. 2007, 101, 1199–1207.
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A Natural Antimicrobial Protein. Int. J. Food Prop. 2019, 22, 1626–1641.
- Silva, P.I.; Daffre, S.; Bulet, P. Isolation and Characterization of Gomesin, an 18-Residue Cysteine-Rich Defense Peptide from the Spider Acanthoscurria gomesiana Hemocytes with Sequence Similarities to Horseshoe Crab Antimicrobial Peptides of the Tachyplesin Family. J. Biol. Chem. 2000, 275, 33464–33470.
- De Lucca, A.J.; Bland, J.M.; Grimm, C.; Jacks, T.J.; Cary, J.W.; Jaynes, J.M.; Cleveland, T.E.; Walsh, T.J. Fungicidal Properties, Sterol Binding, and Proteolytic Resistance of the Synthetic Peptide D4E1. Can. J. Microbiol. 1998, 44, 514–520.
- Zeng, X.-C.; Wang, S.; Nie, Y.; Zhang, L.; Luo, X. Characterization of BmKbpp, a Multifunctional Peptide from the Chinese Scorpion Mesobuthus Martensii Karsch: Gaining Insight into a New Mechanism for the Functional Diversification of Scorpion Venom Peptides. Peptides 2012, 33, 44–51.
- Zhang, Z.-T.; Zhu, S.-Y. Drosomycin, an Essential Component of Antifungal Defence in Drosophila. Insect. Mol. Biol. 2009, 18, 549–556.
- Atanasova-Penichon, V.; Legoahec, L.; Bernillon, S.; Deborde, C.; Maucourt, M.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Ponts, N.; Moing, A.; Richard-Forget, F. Mycotoxin Biosynthesis and Central Metabolism Are Two Interlinked Pathways in Fusarium graminearum, as Demonstrated by the Extensive Metabolic Changes Induced by Caffeic Acid Exposure. Appl. Environ. Microbiol. 2018, 84.
- Lamberty, M.; Zachary, D.; Lanot, R.; Bordereau, C.; Robert, A.; Hoffmann, J.A.; Bulet, P. Insect Immunity. Constitutive Expression of a Cysteine-Rich Antifungal and a Linear Antibacterial Peptide in a Termite Insect. J. Biol. Chem. 2001, 276, 4085–4092.
- Tonk, M.; Cabezas-Cruz, A.; Valdés, J.J.; Rego, R.O.M.; Grubhoffer, L.; Estrada-Peña, A.; Vilcinskas, A.; Kotsyfakis, M.; Rahnamaeian, M. Ixodes ricinus Defensins Attack Distantly-Related Pathogens. Dev. Comp. Immunol. 2015, 53, 358–365.
- Moerman, L.; Bosteels, S.; Noppe, W.; Willems, J.; Clynen, E.; Schoofs, L.; Thevissen, K.; Tytgat, J.; Van Eldere, J.; Van Der Walt, J.; et al. Antibacterial and Antifungal Properties of Alpha-Helical, Cationic Peptides in the Venom of Scorpions from Southern Africa. Eur. J. Biochem. 2002, 269, 4799–4810.
- Destoumieux-Garzón, D.; Rosa, R.D.; Schmitt, P.; Barreto, C.; Vidal-Dupiol, J.; Mitta, G.; Gueguen, Y.; Bachère, E. Antimicrobial Peptides in Marine Invertebrate Health and Disease. Phil. Trans. R. Soc. B 2016, 371, 20150300.
- Lamberty, M.; Caille, A.; Landon, C.; Tassin-Moindrot, S.; Hetru, C.; Bulet, P.; Vovelle, F. Solution Structures of the Antifungal Heliomicin and a Selected Variant with Both Antibacterial and Antifungal Activities. Biochemistry 2001, 40, 11995–12003.
- Zare-Zardini, H.; Taheri-Kafrani, A.; Ordooei, M.; Ebrahimi, L.; Tolueinia, B.; Soleimanizadeh, M. Identification and Biochemical Characterization of a New Antibacterial and Antifungal Peptide Derived from the Insect Sphodromantis viridis. Biochemistry 2015, 80, 433–440.
- Vouldoukis, I.; Shai, Y.; Nicolas, P.; Mor, A. Broad Spectrum Antibiotic Activity of the Skin-PYY. FEBS Lett 1996, 380, 237–240.
- Benincasa, M.; Scocchi, M.; Pacor, S.; Tossi, A.; Nobili, D.; Basaglia, G.; Busetti, M.; Gennaro, R. Fungicidal Activity of Five Cathelicidin Peptides against Clinically Isolated Yeasts. J. Antimicrob. Chemother. 2006, 58, 950–959.
- Lee, D.G.; Kim, H.K.; Kim, S.A.; Park, Y.; Park, S.-C.; Jang, S.-H.; Hahm, K.-S. Fungicidal Effect of Indolicidin and Its Interaction with Phospholipid Membranes. Biochem. Biophys. Res. Commun. 2003, 305, 305–310.
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver*. J. Biol. Chem. 2001, 276, 7806–7810.
- De Lucca, A.J.; Walsh, T.J. Antifungal Peptides: Novel Therapeutic Compounds against Emerging Pathogens. Antimicrob. Agents Chemother. 1999, 43, 1–11.
- Leannec-Rialland, V.; Cabezas-Cruz, A.; Atanasova, V.; Chereau, S.; Ponts, N.; Tonk, M.; Vilcinskas, A.; Ferrer, N.; Valdés, J.J.; Richard-Forget, F. Tick Defensin γ-Core Reduces Fusarium graminearum Growth and Abrogates Mycotoxins Production with High Efficiency. Sci. Rep. 2021, 11, 7962.
- Rajasekaran, K.; Cary, J.W.; Chlan, C.A.; Jaynes, J.M.; Bhatnagar, D. Strategies for Controlling Plant Diseases and Mycotoxin Contamination Using Antimicrobial Synthetic Peptides. In ACS Symposium Series; Rajasekaran, K., Cary, J.W., Jaynes, J.M., Montesinos, E., Eds.; American Chemical Society: Washington, DC, USA, 2012; Volume 1095, pp. 295–315. ISBN 978-0-8412-2748-4.
- López-García, B.; González-Candelas, L.; Pérez-Payá, E.; Marcos, J.F. Identification and Characterization of a Hexapeptide with Activity against Phytopathogenic Fungi That Cause Postharvest Decay in Fruits. Mol. Plant. Microbe Interact. 2000, 13, 837–846.
- López-García, B.; Pérez-Payá, E.; Marcos, J.F. Identification of Novel Hexapeptides Bioactive against Phytopathogenic Fungi through Screening of a Synthetic Peptide Combinatorial Library. Appl Environ. Microbiol. 2002, 68, 2453–2460.
- Muñoz, A.; López-García, B.; Marcos, J.F. Studies on the Mode of Action of the Antifungal Hexapeptide PAF26. Antimicrob. Agents Chemother. 2006, 50, 3847–3855.
- Muñoz, A.; Gandía, M.; Harries, E.; Carmona, L.; Read, N.D.; Marcos, J.F. Understanding the Mechanism of Action of Cell-Penetrating Antifungal Peptides Using the Rationally Designed Hexapeptide PAF26 as a Model. Fungal Biol. Rev. 2013, 26, 146–155.
- Hauser, C.; Thielmann, J.; Muranyi, P. Organic Acids: Usage and Potential in Antimicrobial Packaging. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; Chapter 46; pp. 563–580. ISBN 978-0-12-800723-5.
- Jang, W.S.; Kim, H.K.; Lee, K.Y.; Kim, S.A.; Han, Y.S.; Lee, I.H. Antifungal Activity of Synthetic Peptide Derived from Halocidin, Antimicrobial Peptide from the Tunicate, Halocynthia aurantium. FEBS Lett. 2006, 580, 1490–1496.
- Cary, J.W.; Rajasekaran, K.; Jaynes, J.M.; Cleveland, T.E. Transgenic Expression of a Gene Encoding a Synthetic Antimicrobial Peptide Results in Inhibition of Fungal Growth in Vitro and in Planta. Plant. Sci. 2000, 154, 171–181.
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.P.; Roussel, J.P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-Activity Analysis of Thanatin, a 21-Residue Inducible Insect Defense Peptide with Sequence Homology to Frog Skin Antimicrobial Peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 1221–1225.
- Osusky, M.; Zhou, G.; Osuska, L.; Hancock, R.E.W.; Kay, W.; Misra, S. Transgenic Plants Expressing Cationic Peptide Chimeras Exhibit Broad-Spectrum Resistance to Phytopathogens. Nat. Biotechnol. 2000, 18, 1162–1166.
- Badosa, E.; Ferre, R.; Francés, J.; Bardají, E.; Feliu, L.; Planas, M.; Montesinos, E. Sporicidal Activity of Synthetic Antifungal Undecapeptides and Control of Penicillium Rot of Apples. Appl. Environ. Microbiol. 2009, 75, 5563–5569.
- Jiang, Z.; Kullberg, B.J.; van der Lee, H.; Vasil, A.I.; Hale, J.D.; Mant, C.T.; Hancock, R.E.W.; Vasil, M.L.; Netea, M.G.; Hodges, R.S. Effects of Hydrophobicity on the Antifungal Activity of α-Helical Antimicrobial Peptides. Chem. Biol. Drug Des. 2008, 72, 483–495.
- Ramamourthy, G.; Na, H.; Seo, C.; Park, Y. Antifungal Activity of (KW)n or (RW)n Peptide against Fusarium solani and Fusarium oxysporum. Int. J. Mol. Sci. 2012, 13, 15042–15053.
- Thery, T.; O’Callaghan, Y.; O’Brien, N.; Arendt, E.K. Optimisation of the Antifungal Potency of the Amidated Peptide H-Orn-Orn-Trp-Trp-NH2 against Food Contaminants. Int. J. Food Microbiol. 2018, 265, 40–48.
- Reed, J.D.; Edwards, D.L.; Gonzalez, C.F. Synthetic Peptide Combinatorial Libraries: A Method for the Identification of Bioactive Peptides Against Phytopathogenic Fungi. MPMI 1997, 10, 537–549.
- Devi, M.S.; Sashidhar, R.B. Antiaflatoxigenic Effects of Selected Antifungal Peptides. Peptides 2019, 115, 15–26.
