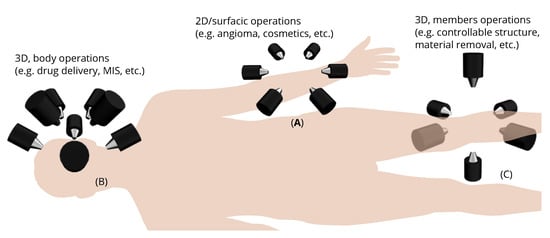
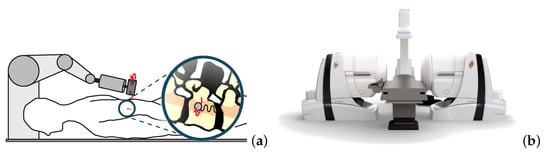
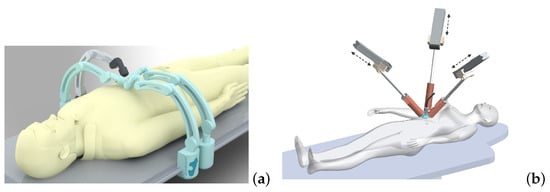
Magnetic microrobotics is a promising technology for improving minimally invasive surgery (MIS) with the ambition of enhancing patient care and comfort. The potential benefits include limited incisions, less hemorrhaging and postoperative pain, and faster recovery time. To achieve this, a key issue relies on the design of a proper electromagnetic actuation (EMA) setup which is based on the use of magnetic sources. The magnetic field and its gradient generated by the EMA platform is then used to induce magnetic torque and force for microrobot manipulations inside the human body. Like any control systems, the EMA system must be adapted to the given controlled microrobot and customized for the application.
- electromagnetic actuation
- medical magnetic microrobots
- minimally invasive surgery
1. Introduction
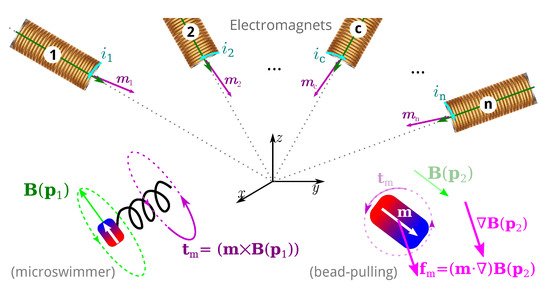
2. Theoretical Background
2.1. Magnetic Manipulation
EMA systems consisting of several electromagnets allow generating a magnetic field and/or a gradient field in a given workspace, as shown in Figure 2. These fields induce a magnetic torque and a force on the magnetized materials of the untethered microrobots. The expression of the magnetic field generated by an electromagnetic coil is derived from a single wire and the magnetic dipole. The magnetic field from any electromagnetic coil c can be approximated as a magnetic dipole characterized by its magnetic moment mc, and the point-dipole model is proposed. It can be shown that the magnetic field and its gradient are proportional to the electric current ic flowing through the coil c. The overall magnetic field generated by the n-coils is the superposition of each field. The magnetic field and its gradient are then expressed as:

where i=(i1,…,in)t is the electric current vector.

where A(m,p)∈R6×n is an actuation matrix mapping the current to the applied magnetic wrench. This magnetic actuation matrix is a function of both the position p∈Ω, and the magnetic moment m of the microrobot.
Therefore, substituting Equations (1) and (2) into Equation (3), the equations of torque and force can be presented by the actuation matrix A(m,p) in the further details as:
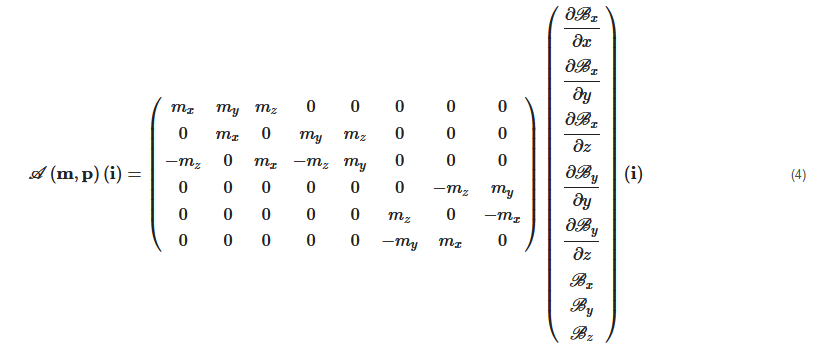
Each column of the matrix A(m,p) represents the wrench on the force and torque per unit current created by each electromagnet. If there are greater than n>6 electromagnets, the actuation matrix A(m,p) leads to a better conditioned matrix, a more isotropic workspace Ω, a reduction of singularity configurations, and lower current requirements [72][73][81][72,73,81]. In such cases, n>6, the EMA system is said "redundant" for the task. Especially, if A(m,p) has a full rank, for a desired force, f★m and torque, t★m, the actuation currents i can be calculated from the pseudo-inverse:

If n<6, the pseudo-inverse would be a least-squares approximation. Hence, for a controlled force and torque, the input current can be obtained only if the pseudo-inverse of A(m,p) exists. This derivation on the controlled current i can be similarly extended for controllers that require torque and/or force control [57].
2.2. Manipulation Analysis
From the mathematical analysis, the rank of force equation is 3 and the rank of torque equation is 2, the microrobot can maximally achieve three degrees-of-freedom (DOFs) in translation and two DOFs in rotation. Next, to achieve the five DOFs control of the microrobot, the minimum number of electromagnets is mathematical estimated. The three electromagnets can be used for three DOFs force control at a point, but normally five electromagnets are required when the orientation of the microrobot is dynamic changed. The number of electromagnets can be reduced to four, but either a nonmagnetic restoring torque or a nonmagnetic restoring force is required to stabilize the system. For two DOFs torque control, only three electromagnets are required because the three coils can generate a 3D field in a workspace. Thus, combined torque and force control requires a minimum of n=7 stationary electromagnets. Similarly, the seven electromagnets also need some additional external conditions. To stabilize the five DOFs control of the microrobot, the eight electromagnets are suggested for the fixed configuration system [83]. Reconfigurable EMA system can achieve similar control authority to stationary system with fewer electromagnets. Only n=5 electromagnets are required for torque and force control. Therefore, the mobile electromagnets are more particularly considered for the biomedical applications. Indeed, the field shape in the workspace can be modified by changing the location or orientation of the electromagnets during the magnetic actuation of the microrobot
[81].
2.3. Discussions
Various arrangements of electromagnetic coils can generate various magnetic field distributions. The EMA setup should be properly defined with respect to the envisioned biomedical application. To do so, the main characteristics should be specified, such as: the environment of the workspace, the type of microrobot and the various magnetic tasks. The required number of electromagnets for different motions control has been studied in past works [45][81][83][45,81,83]. On this basis, the relations between the specifications and the number of coils to design an EMA system can be proposed, and are depicted in Figure 3.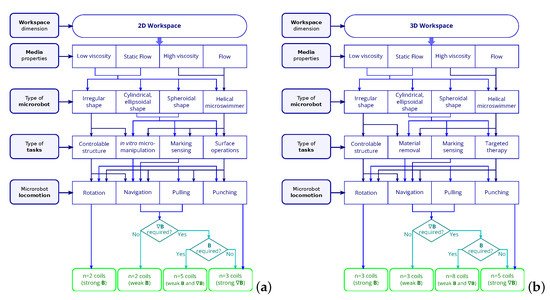
-
The dimensions of workspace;
-
The media of the environment;
-
The type of microrobots;
-
The medical tasks;
-
The required motion control.
Finally, the number of electromagnetic coils is determined by the specific motions of the selected microrobot. For instance, the translational locomotion can be achieved by the magnetic force on the spheroidal microrobot, and it can also be reached by the magnetic torque generated by rotating magnetic field on the helical microrobot.
3. The Electromagnetic Microrobotic Systems
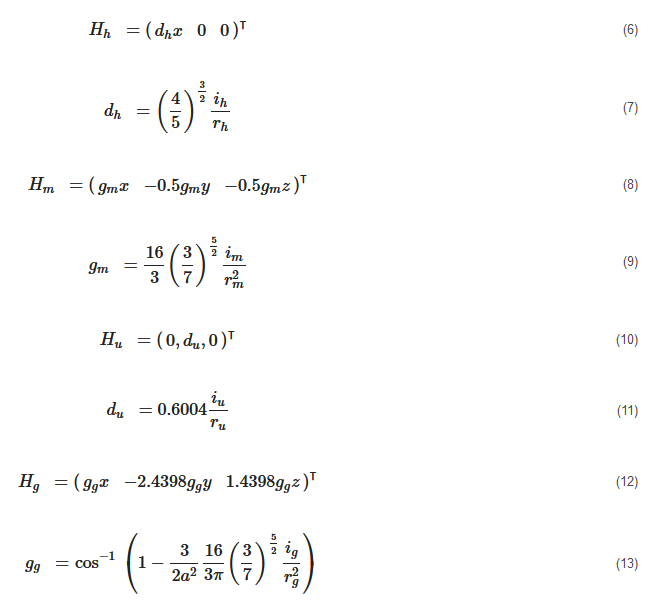
where ih and rh=r are the current and the radius of the Helmholtz coils; im and rm=r are the current and the radius of the Maxwell coils; iu and ru=r are the current and the radius of the uniform saddle coils; and ig and ru=g are the current and the radius of the gradient saddle coils.
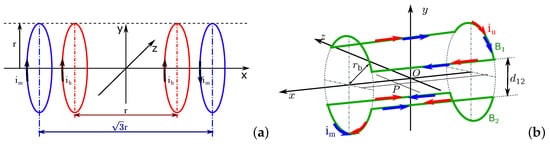
As shown in Figure 6a, the Helmholtz set includes two solenoids with same radius rh separated by the distance: l=rh, and the Maxwell coil consists of a pair of same coils of radius rm separated with a distance l=3–√rm. The currents flowing in an Helmholtz coil pair have same intensity and phase, that is: ih=ihleft=ihright, while the currents of Maxwell coils are flowing in opposite phases, that is: imleft=−imright and im=|imleft|=|imright∣∣. It is clear that the magnetic fields generated by the combination of a Helmholtz coils pair and a Maxwell coils pair are different from that produced by two Helmholtz coils pairs. Hence, the different configurations of platforms composed of different coils pairs will be investigated. To make it easier to name each magnetic platform, the researchers introduce the abbreviation to identify them with the nomenclature provided in Table 1.
Table 1. ElectroMagnetic Actuation system nomenclature.
EMA | Coils and Workspace | Advantages | Limitations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
HMr [49] | 4 coils, 2D | Use few coils with simple structure; 2 DOFs of translation and 1 DOF of rotation; Manipulation of the orthotropic body. | Low magnetic gradient over larger areas; Rotating coils limit medical applications. | ||||||||
2C [70] | 4 coils, 2D | Less number of coils, 18% smaller volume and 26.7% less power consumption to the same magnetic actuation. | Non-uniform magnetic field at the edge of the workspace. | ||||||||
2H2M [65] | Eight coils, 2D | Simple current control strategy; Uniform magnetic field and gradient; Manipulation of the orthotropic body. | Low flexibility of the confined workspace. | ||||||||
HMUG [62] | Eight coils, 2D | Common current control strategy; Compact setup with easy access; More efficient compared to the Maxwell and Helmholtz coils; Manipulation of the orthotropic body. | Size of the proposed workspace is still limited; Non-uniform magnetic field at the edge of the workspace; Effective workspace is limited to the 2D plane. | ||||||||
Ten coils, 2D | Independent control of the multiple microrobots; Microparticles with different shapes. | Only 2 DOFs motions (1 DOF rotational and 1 DOF translational) on a 2D plane; Confined workspace with difficult access; Important number of coils and power consumption. | |||||||||
6E [92] | Six coils, 2D | Simultaneous independent positioning control of multiple microrobots; Simple structure and easy to implement; Heterogeneous sets of dissimilar magnetic microrobots have been tested. | Complex control algorithms, and limited trajectory. | ||||||||
3DMH [74] | Six coils, 3D | Compact and cheap setup; 3D manipulation of the orthotropic body. | Limited workspace. | ||||||||
2H2Mr [61] | Eight coils, 3D | Precise 3D motion; Fewer number of coils; Manipulation of the orthotropic body. | Rotating coils limit medical applications; Large setup volume and power consumption. | ||||||||
HMUGr [67] | Eight coils, 3D | Small setup volume and less consumption; Workspace accessibility; Almost all kind of microrobots. | More powerful current suppliers are demanded that will cause overheating; The available workspace is still relatively small. | ||||||||
H2US-MUG [63] | Ten coils, 3D | Compact structure; Precise magnetic field and gradient for 3D manipulation; Potential large range of applications. | Large number of coils; Small and limited size. | ||||||||
3DH [93] | [94] |
Six coils, 3D | 6 DOFs motion; Simple structure and easy to build; Control of the helical microswimmer. | Complex algorithm and control strategy; Only the magnetic-field-based control. | |||||||
8E [94] | [95] |
Eight coils, 3D | Compact structure; Arbitrary forces can be exerted on each microrobot independently and simultaneously. | Small volume with few accessibility; Weak magnetic field and gradients. | |||||||
Good magnetic field; Hemispherical coils arrangement. | Complex magnetic field description. | ||||||||||
9E [95] | [96] |
Nine coils, 3D | Independent 3D control of pairs of microrobots; 6 DOFs motion; Workspace accessibility. | Advanced control strategies are required; Large amount of energy consumption and heating. |
Symbol |
|---|
Description | Symbol | Description | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
H | Helmholtz coils | M | Maxwell coils | ||||||||
U | Uniform saddle coils | G | Gradient saddle coils | ||||||||
E | Electromagnet | C | single coil pair | ||||||||
2D | two-dimensions | 3D | three-dimensions | ||||||||
r | rotational |
