Ibrexafungerp is a first-in-class IV/oral triterpenoid antifungal agent. Similar in mechanism of action to echinocandins, ibrexafungerp inhibits (1→3)-β-D-glucan synthase, a key component of the fungal cell wall, resulting in fungicidal activity against
Definition:
Ibrexafungerp is a first-in-class IV/oral triterpenoid antifungal agent. Similar in mechanism of action to echinocandins, ibrexafungerp inhibits (1→3)-β-D-glucan synthase, a key component of the fungal cell wall, resulting in fungicidal activity against
Candida
spp. Ibrexafungerp demonstrates broad
in vitro
activity against
Candida
spp.,Aspergillus spp., dimorphic fungi Pneumocystis and other emerging yeasts and mold pathogens including azole and echinocandin-resistant isolates. It is currently in late clinical development for treatment and prevention of vulvovaginal candidiasis. Other ongoing trials include treatment of serious fungal infections, including, invasive candidiasis,
Candida auris infections, invasive aspergillosis and refractory fungal disease in patients not responding to or who are intolerant to standard of care .
infections, invasive aspergillosis and refractory fungal disease in patients not responding to or who are intolerant to standard of care .
- Ibrexafungerp
- SCY-078
- Glucan syntheses inhibitor
- Candida auris
1. Ibrexafungerp
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Ibrexafungerp
1.1. Mechanism of Action
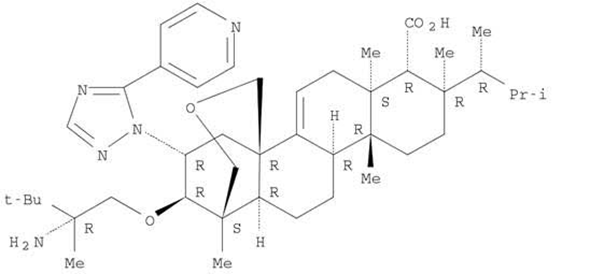
Ibrexafungerp (formerly SCY-078) is the first compound of the enfumafungin-derived triterpenoid class of (1→3)-β-D-glucan synthase inhibitors (GSIs) (Figure 1). Glucan synthase inhibitors were first introduced for the treatment of invasive
Candida infections in 2001, with caspofungin the first echinocandin to be approved [1]. This mechanism of action, i.e., blockade of the biosynthesis of ß-(1,3)-D-glucan in the fungal cell wall, was associated with potent and broad-spectrum antifungal activity and clinical efficacy for the treatment of fungal infections. Two additional echinocandins were later introduced, micafungin and anidulafungin. However, echinocandins lack clinically meaningful oral bioavailability, triggering the search for new molecules that shared the glucan synthase inhibition mechanism of action with echinocandins and could also be administered orally. Natural screening efforts led to the identification of enfumafungin derivatives as candidates, and subsequent synthetic modifications to these molecules resulted in increased oral bioavailability, potency, and stability, thereby leading to the discovery of ibrexafungerp.
infections in 2001, with caspofungin the first echinocandin to be approved [19]. This mechanism of action, i.e., blockade of the biosynthesis of ß-(1,3)-D-glucan in the fungal cell wall, was associated with potent and broad-spectrum antifungal activity and clinical efficacy for the treatment of fungal infections. Two additional echinocandins were later introduced, micafungin and anidulafungin. However, echinocandins lack clinically meaningful oral bioavailability, triggering the search for new molecules that shared the glucan synthase inhibition mechanism of action with echinocandins and could also be administered orally. Natural screening efforts led to the identification of enfumafungin derivatives as candidates, and subsequent synthetic modifications to these molecules resulted in increased oral bioavailability, potency, and stability, thereby leading to the discovery of ibrexafungerp.
Figure 1.
Structure of ibrexafungerp.
Ibrexafungerp is being developed as the first oral and IV GSI (Intravenous glucan synthase inhibitor) for the treatment and prevention of fungal infections, including serious and life-threatening infections due to
Candida
spp.,
Aspergillus
spp., and
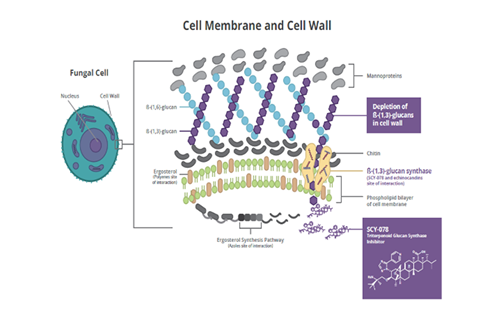
Pneumocystis jirovecii, with the potential to provide the therapeutic advantages of both IV and oral formulations [2]. Ibrexafungerp causes a decrease in (1→3)-β-D-glucan polymers and a weakening of the fungal cell wall [3]. Ibrexafungerp is structurally distinct from echinocandins and interacts differently with the target enzyme (Figure 2) [4]. Although the binding site on (1→3)-β-D-glucan synthase for ibrexafungerp partially overlaps with a binding site for echinocandins, it appears to be nonidentical, resulting in a lower rate of resistance to ibrexafungerp [4]. In in vitro studies, ibrexafungerp activity against wild-type and echinocandin-resistant strains of
, with the potential to provide the therapeutic advantages of both IV and oral formulations [20]. Ibrexafungerp causes a decrease in (1→3)-β-D-glucan polymers and a weakening of the fungal cell wall [21]. Ibrexafungerp is structurally distinct from echinocandins and interacts differently with the target enzyme (Figure 2) [22]. Although the binding site on (1→3)-β-D-glucan synthase for ibrexafungerp partially overlaps with a binding site for echinocandins, it appears to be nonidentical, resulting in a lower rate of resistance to ibrexafungerp [22]. In in vitro studies, ibrexafungerp activity against wild-type and echinocandin-resistant strains of
Candida
spp. in the presence of
fks mutations was minimally affected [5]. Thus, ibrexafungerp has limited potential for cross-resistance with echinocandins.
mutations was minimally affected [23]. Thus, ibrexafungerp has limited potential for cross-resistance with echinocandins.
Figure 2.
Mechanism of action for ibrexafungerp.
1.2 Pharmacokinetics/Pharmacodynamics
Ibrexafungerp is being developed for both oral and IV dosing. Oral ibrexafungerp has a bioavailability of approximately 30% that is optimized if taken with meals which increase it by approximately 40%. The half-life of approximately 20 hours and the PK/PD driver of antifungal activity is AUC/MIC. It is characterized by high-volume distribution and extensive tissue penetration though it does not achieve central nervous system (CNS) penetration. It met efficacy end points across multiple murine models of invasive candidiasis at concentrations that have been safely achieved after oral administration in humans. An murine intra-abdominal candidiasis model study showed robust drug penetration at the site of infection for Intra-abdominal Candidiasis. Drug concentrations within the necrotic center of liver abscesses are almost 100-fold higher than the serum concentrations at corresponding time points. Ibrexafungerp exhibits pH-dependent solubility, achieving the highest concentrations in acidic media consistent with simulated gastric and intestinal fluids, which may increase its efficacy in vaginal tissues as well as in abscesses. Ibrexafungerp accumulates in the vaginal tissue in mice with plasma to tissue ratio of 1 to 9.
In vitro
and drug-drug interaction studies in humans, suggesting low risk for significant CYP450 drug-drug interactions with ibrexafungerp.
1.3 In vitro and In vivo Activity
In vitro
and i
n vivo
studies have demonstrated potency against the most common
Candida
spp, as well as resistant isolates. In addition, it demonstrates excellent
in vitro
activity against wild-type and azole-resistant strains of
Aspergillus
spp,
Paecilomyces variotii
, and some activity against
L prolificans
, but poor activity was observed against
Mucor
and
Fusarium
spp. It retains activity against
Candida
FKS1 or FKS2 mutants, including echinocandin-resistant isolates of
C glabrata
and
C auris.
Due to differential binding sites, the FKS mutations associated with echinocandin resistance are distinctly different from those reducing susceptibility to the enfumafungin derivatives including ibrexafungerp, limiting cross-resistance. By both CLSI and EUCAST broth microdilution methodologies, MICs were determined to be 0.06–2 mg/L against
C albicans
and
C tropicalis
, 0.25–1 mg/L against
C parapsilosis
, and 0.5–2 mg/L against
C glabrata
and
C krusei
. Against
Aspergillus
spp, MEC values reported were 0.03–1 mg/L and 0.015–0.25 mg/L when tested by CLSI and EUCAST methods, respectively. Studies also show that oral ibrexafungerp could be a viable option for managing
Pneumocystis
in immunocompromised patients, because it has shown significant activity in a murine therapy and prophylaxis model.
1.4 Stage of Development and Ongoing Clinical Studies
Ibrexafungerp has completed two phase 3 studies in vulvovaginal candidiasis and three phase 2 studies, two in VVC and one in invasive candidiasis. Ibrexafungerp met primary end points in two phase 2 clinical trials (NCT02679456, NCT02244606) in VVC and invasive candidiasis, respectively. In VVC, oral ibrexafungerp was superior compared with oral fluconazole (78% vs 65%), and at the end of a 4-month follow-up period (88% vs 65%) with a lower recurrence rate (4% vs 15%). For invasive candidiasis, ibrexafungerp, as an oral step-down treatment after 3–10 days of echinocandin therapy, compared with fluconazole, achieved the predetermined target exposure in most subjects. Mild-to-moderate gastrointestinal events such as diarrhea, nausea, vomiting, abdominal pain or discomfort were seen with ibrexafungerp, but no discontinuations due to AEs or serious AEs were observed. No mycological failure was reported in the study drug arm. A recently completed phase 2 trial, the DOVE study (NCT03253094), explored 5 dosing regimens of oral ibrexafungerp versus fluconazole in patients with acute VVC to identify an optimal dose for a phase 3 trial. Two identical phase 3 studies, VANISH-303 (US) (NCT03734991) and VANISH-306 (US and EU) (NCT03987620) were performed in female patients with VVC. These multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of oral ibrexafungerp (SCY-078) vs. placebo in subjects with acute vulvovaginal candidiasis compared ibrexafungerp 300 mg BID for one day versus placebo in a 2:1 randomization. Both studies demonstrated superiority of ibrexafungerp versus placebo in both primary and secondary endpoints.
Currently, there are multiple ongoing phase 3 trials. The CARES study (NCT03363841), open for enrollment in the United States and India, is assessing ibrexafungerp for the treatment of
C auris
infections. Initial evidence of efficacy and safety in 2 subjects was presented with promising results. The FURI study (NCT03059992) is evaluating the efficacy and safety in patients with an invasive and/or severe fungal disease that are refractory or intolerant to standard-of-care antifungal treatment. Initial results of 41 patients who completed therapy were favorable. The Data Review Committee adjudicated 56% of patients as achieving complete or partial response, 27% maintaining stable disease, 15% with progression of disease, and 1 case was considered as indeterminate. Outcomes for 2 cases of
C albicans and C tropicalis
spondylodiscitis have shown clinical data for its use in bone infections. Ongoing studies will evaluate oral ibrexafungerp in patients with recurrent VVC (CANDLE-304, NCT03987620) and as combination therapy with voriconazole in patients with invasive pulmonary aspergillosis (SCYNERGIA study, NCT03672292).
2.
Candida auris Introduction
Candida auris is an emerging fungal pathogen reported on all continents except Antarctica, in at least 39 countries worldwide [6], as well as in 20 states of the United States [7][8][9][10]. Five distinct clades of
is an emerging fungal pathogen reported on all continents except Antarctica, in at least 39 countries worldwide [1], as well as in 20 states of the United States [2–5]. Five distinct clades of
C. auris were identified with well-defined geographic distributions (South America, Africa, South Asia, East Asia, and West Asia), as well as antifungal resistance patterns and mechanisms that are both distinct and unique [6][11][12][13]. Infections due to
were identified with well-defined geographic distributions (South America, Africa, South Asia, East Asia, and West Asia), as well as antifungal resistance patterns and mechanisms that are both distinct and unique [1,6–8]. Infections due to
C. auris are most often nosocomial, with easy transmission from patient-to-environment and environment-to-patient [11][14]. Patients heavily colonized with
are most often nosocomial, with easy transmission from patient-to-environment and environment-to-patient [6,9]. Patients heavily colonized with
C. auris
on the skin or mucosal surfaces can contaminate their surroundings, thereby contributing to transmission of
C. auris
in healthcare facilities
.
An additional challenge with
C. auris is that the organism is exceedingly difficult to eradicate from the environment because of resistance to some standard disinfectants [11][14][15].
is that the organism is exceedingly difficult to eradicate from the environment because of resistance to some standard disinfectants [6,9,10].
Timely and accurate diagnosis of invasive candidiasis are important for early initiation of antifungal therapy, while species identification is critical to ensure implementation of infection control measures [6][11][15][16]. Acceptable standard diagnostic methods for
Timely and accurate diagnosis of invasive candidiasis are important for early initiation of antifungal therapy, while species identification is critical to ensure implementation of infection control measures [1,6,10,11]. Acceptable standard diagnostic methods for
C. auris identification include matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) and VITEK2™ with the appropriate updated databases and DNA sequencing [12][17].
identification include matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) and VITEK2™ with the appropriate updated databases and DNA sequencing [7,12].
3. Ibrexafungerp for
Candida auris
3.1. In Vitro Activity
The in vitro activity of ibrexafungerp was tested against 16
C. auris clinical isolates obtained from Germany, Japan, India, and South Korea [18]. The MIC
clinical isolates obtained from Germany, Japan, India, and South Korea [27]. The MIC
90
for ibrexafungerp was 1 μg/mL. Fluconazole and amphotericin B exhibited less in vitro activity against
C. auris
with MIC
90
values of >64 and 4 μg/mL, respectively, while the MIC
90
values for anidulafungin, caspofungin, and micafungin were 0.25 μg/mL, 1 μg/mL, and 1 μg/mL, respectively.
The in vitro activity of ibrexafungerp was evaluated against a global collection of 100 isolates of
C. auris
representing each of the four clades of
C. auris known at that time [19]. MICs for ibrexafungerp ranged from 0.0625 to 2 μg/mL, with an MIC
known at that time [24]. MICs for ibrexafungerp ranged from 0.0625 to 2 μg/mL, with an MIC
50
of 0.5 μg/mL and MIC
90
1 μg/mL. MIC values for anidulafungin, caspofungin, and micafungin ranged from 0.03 up to >16 μg/mL. Among seven
C. auris
isolates exhibiting elevated MIC values for echinocandins, the ibrexafungerp MIC ranged from 0.5 to 1.0 μg/mL.
Ibrexafungerp and six comparator antifungal agents were evaluated against 122
C. auris isolates [20]. The MIC range for ibrexafungerp was 0.06 to 2.0 μg/mL. A wide distribution of MIC values was reported for anidulafungin and micafungin, ranging from 0.016 to >32 and 0.03 to >32 μg/mL, respectively (Table 1). All but one
isolates [33]. The MIC range for ibrexafungerp was 0.06 to 2.0 μg/mL. A wide distribution of MIC values was reported for anidulafungin and micafungin, ranging from 0.016 to >32 and 0.03 to >32 μg/mL, respectively (Table 1). All but one
C. auris
isolate were resistant to fluconazole. Out of 122 isolates, 8 displayed high MIC values for echinocandins associated with
fks
mutations (S639F Fks1 alteration). The MIC for ibrexafungerp for these eight resistant isolates ranged from 0.25 to 0.5 μg/mL.
Table 1.
In vitro activity of ibrexafungerp and comparators against
C. auris isolates [20].
isolates [33].
|
Drug (No. of Isolates) |
MIC50 a |
Modal MIC |
MIC Range |
|
Ibrexafungerp (n = 122) |
0.5 |
0.5 |
0.06–2 |
|
Anidulafungin |
0.125 |
0.06 |
0.016–>32 |
|
Micafungin |
0.125 |
0.125 |
0.03–>32 |
|
Amphotericin B |
1 |
1 |
0.5–1 |
|
Fluconazole |
≥64 |
≥64 |
0.5–≥64 |
|
Voriconazole |
0.5 |
Bimodal |
≤0.004–4 |
|
Isavuconazole |
0.125 |
Trimodal |
≤0.004–2 |
a
μg/mL; Ibrexafungerp minimum inhibitory concentration (MIC) values for eight isolates with S639F
fks1
mutations ranged from 0.25 to 0.5 μg/mL.
Among 102
C. auris
isolates with variable resistance to amphotericin B, flucytosine, azoles, and echinocandins, the ibrexafungerp MIC
50 for 97 isolates ranged from 0.06–0.5 μg/mL, and the median and mode MIC were both 0.5 μg/mL [21]. Ibrexafungerp also showed activity against five
for 97 isolates ranged from 0.06–0.5 μg/mL, and the median and mode MIC were both 0.5 μg/mL [32]. Ibrexafungerp also showed activity against five
C. auris
isolates considered to be pan-resistant, with a low MIC
50
range of 0.12 to 1 μg/mL.
Data were compiled from four studies reporting the in vitro activity of ibrexafungerp against 445
C. auris clinical isolates [22]. Most isolates were obtained from the United States and India and included 32 isolates with increased MIC values to echinocandins. The MIC
clinical isolates [47]. Most isolates were obtained from the United States and India and included 32 isolates with increased MIC values to echinocandins. The MIC
50
and MIC
90
for ibrexafungerp across all isolates tested were 0.5 μg/mL and 1.0 μg/mL, respectively (Table 2). Among 32
C. auris
isolates with echinocandin resistance, MIC values for ibrexafungerp ranged from 0.5 μg/mL to 1.0 μg/mL. One isolate displayed high MIC values for echinocandins and showed reduced sensitivity (>2 dilutions vs. the mode) to ibrexafungerp, and this isolate exhibited elevated MIC values to anidulafungin, caspofungin, and micafungin (MIC = 1 μg/mL), luconazole (MIC > 256 μg/mL), and amphotericin B (MIC = 1 μg/mL). Thus, ibrexafungerp exhibits in vitro activity against a broad collection of
C. auris
isolates, including most echinocandin-resistant isolates.
Table 2.
In vitro activity of ibrexafungerp against a compilation of 445
C. auris isolates [22].
isolates [47].
|
Reference |
No. of Isolates |
MIC, μg/mL |
|||
|
MIC50 |
MIC90 |
Mode |
MIC Range |
||
|
Berkow et al., 2017 [19] |
107 |
1 |
1 |
1 |
0.0625–2 |
|
Larkin et al., 2017 [18] |
16 |
1 |
1 |
1 |
0.5–1 |
|
Zhu et al., 2020 [21] |
200 |
0.5 |
1 |
0.5 |
0.0625–8 |
|
Arendrup et al., 2020 [20] |
122 |
0.5 |
1 |
0.5 |
0.0625–2 |
|
Overall |
445 |
0.5 |
1 |
0.5 |
0.625–8 |
|
Reference |
No. of Isolates |
MIC, μg/mL |
|||
|
MIC50 |
MIC90 |
Mode |
MIC Range |
||
|
Berkow et al., 2017 [24] |
107 |
1 |
1 |
1 |
0.0625–2 |
|
Larkin et al., 2017 [27] |
16 |
1 |
1 |
1 |
0.5–1 |
|
Zhu et al., 2020 [32] |
200 |
0.5 |
1 |
0.5 |
0.0625–8 |
|
Arendrup et al., 2020 [33] |
122 |
0.5 |
1 |
0.5 |
0.0625–2 |
|
Overall |
445 |
0.5 |
1 |
0.5 |
0.625–8 |
The ability of
Candida
species to form biofilms is associated with catheter and device-related infections and may play a role in
C. auris
infections considering that many affected individuals are in intensive care units with intravascular lines. In this regard, 97% of patients infected with
C. auris had central venous catheters (Sayeed et al., 2019) [23], and a retrospective analysis demonstrated significantly higher use of central venous catheters in patients infected with this multidrug- resistant
had central venous catheters (Sayeed et al., 2019) [48], and a retrospective analysis demonstrated significantly higher use of central venous catheters in patients infected with this multidrug- resistant
Candida [24]. The activity of ibrexafungerp against
[49]. The activity of ibrexafungerp against
C. auris biofilms was evaluated [[18]. Following 48 h of incubation, metabolic activities of biofilms were measured. Images and thicknesses of biofilms growing in the presence or absence of a drug were captured using confocal scanning laser microscopy. Quantitation of the metabolic activity of
biofilms was evaluated [27]. Following 48 h of incubation, metabolic activities of biofilms were measured. Images and thicknesses of biofilms growing in the presence or absence of a drug were captured using confocal scanning laser microscopy. Quantitation of the metabolic activity of
C. auris biofilms was performed using a biochemical assay, the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay, as described previously [18][25]. Ibrexafungerp demonstrated activity against
biofilms was performed using a biochemical assay, the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay, as described previously [27,50]. Ibrexafungerp demonstrated activity against
C. auris
biofilms by reducing biofilm thickness and metabolic activity.
The effects of ibrexafungerp and caspofungin on the morphology of
C. albicans
,
C. auris
, and
C. glabrata were studied using scanning and transmission electron microscopy [26]. When evaluated at respective MIC
were studied using scanning and transmission electron microscopy [51]. When evaluated at respective MIC
50
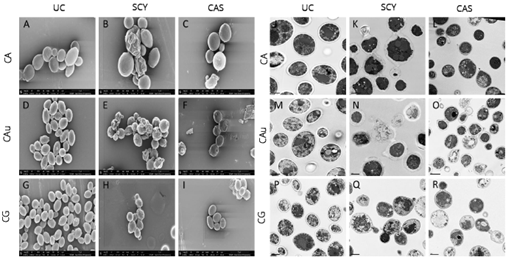
levels, ibrexafungerp exhibited a profound effect on cellular morphology in caspofungin-resistant organisms, possibly indicative of a difference in target engagement between ibrexafungerp and echinocandins (Figure 3). Untreated control
C. auris cells showed well-defined, oval-shaped yeast morphology, as well as several budding yeasts. In contrast, cells exposed to ibrexafungerp (at a concentration of 1 µL MIC) exhibited a severely distorted yeast cell topography, including cell collapse, deformed cellular appearance, irregular budding, and cells that were fused together and unable to undergo cell division [18].
cells showed well-defined, oval-shaped yeast morphology, as well as several budding yeasts. In contrast, cells exposed to ibrexafungerp (at a concentration of 1 µL MIC) exhibited a severely distorted yeast cell topography, including cell collapse, deformed cellular appearance, irregular budding, and cells that were fused together and unable to undergo cell division [27].
Figure 3.
Scanning electron microscopy of untreated cells (panels (
A
,
D
,
G
)), cells treated with ibrexafungerp (panels (
B
,
E
,
H
)), and cells treated with caspofungin (panels (
C
,
F
,
I)) (Hager et al., 2018) [26].
)) (Hager et al., 2018) [51].
3.2. In Vivo Activity
The in vivo efficacy of ibrexafungerp for
C. auris was evaluated in a disseminated murine mouse model [27]. Immunocompromised mice were randomized to ibrexafungerp 10, 20, or 30 mg/kg twice daily (BID) vs. a vehicle given by oral gavage. At Day 7, the fungal burden in kidney tissue was reduced by all doses of ibrexafungerp, with a significant difference for the 30 mg/kg dose vs. vehicle. At Day 14, survival rates were 60–70% with ibrexafungerp vs. 20% with vehicle control. Exposures in mice dosed with ibrexafungerp 10, 20, or 30 mg/kg BID were consistent with steady-state plasma exposure (AUC
was evaluated in a disseminated murine mouse model [37]. Immunocompromised mice were randomized to ibrexafungerp 10, 20, or 30 mg/kg twice daily (BID) vs. a vehicle given by oral gavage. At Day 7, the fungal burden in kidney tissue was reduced by all doses of ibrexafungerp, with a significant difference for the 30 mg/kg dose vs. vehicle. At Day 14, survival rates were 60–70% with ibrexafungerp vs. 20% with vehicle control. Exposures in mice dosed with ibrexafungerp 10, 20, or 30 mg/kg BID were consistent with steady-state plasma exposure (AUC
0–24
) of 8.4, 24.3, and 40.2 ug*h/mL, respectively. These results demonstrate potent antifungal activity of ibrexafungerp against
C. auris
.
C. auris
colonization is a major problem in hospitals and long-term care facilities. In order to understand the ability of ibrexafungerp to potentially decolonize the skin of
C. auris, a study was performed looking at the in vivo efficacy of ibrexafungerp in a cutaneous infection model in Guinea pigs [28]. Animals were treated with ibrexafungerp 10, 20, or 30 mg/kg BID by oral gavage, micafungin 5 mg/kg once daily IP, or vehicle by oral gavage, and prednisone 30 mg/kg SC was given one day before and three days after infection. Tissue burden at Day 7 was lower with all active treatments vs. vehicle. Animals dosed with ibrexafungerp 10, 20, or 30 mg/kg BID showed systemic exposures (AUC
, a study was performed looking at the in vivo efficacy of ibrexafungerp in a cutaneous infection model in Guinea pigs [36]. Animals were treated with ibrexafungerp 10, 20, or 30 mg/kg BID by oral gavage, micafungin 5 mg/kg once daily IP, or vehicle by oral gavage, and prednisone 30 mg/kg SC was given one day before and three days after infection. Tissue burden at Day 7 was lower with all active treatments vs. vehicle. Animals dosed with ibrexafungerp 10, 20, or 30 mg/kg BID showed systemic exposures (AUC
0–24
) of 2.8, 5.6, and 15 ug*h/mL. Examination of Periodic Acid-Schiff (PAS)-stained skin sections revealed that sections obtained from untreated control animals showed yeast cells, demonstrating that the skin was infected with
C. auris
. In contrast, examination of multiple skin sections obtained from animals treated with either ibrexafungerp or micafungin did not reveal yeast cells at any of the dose levels tested, indicating that the
C. auris
infection was cleared. There was no significant difference in clinical scores between the treatment groups [36]. Thus, no fungal elements were observed with ibrexafungerp or micafungin from histological examination.
3.3. Clinical Experience
CARES is an open-label study of oral ibrexafungerp in patients with documented candidiasis or candidemia due to
C. auris
who were treatment naïve or refractory to or intolerant of standard-of-care antifungal agents (clinicaltrials.gov: NCT03363841). Patients were treated with oral ibrexafungerp 750 mg twice daily for two days, then 750 mg once daily for up to 90 days.
In the first two patients from CARES with candidemia due to
C. auris
, a complete response after 17 and 22 days of treatment was reported with ibrexafungerp [52]. The first patient was a 58-year-old male admitted to the ICU with pneumonia and septic shock. Antibiotics were given together with empiric IV fluconazole. When
C. auris
was isolated from blood cultures, antifungal therapy was switched to IV micafungin. However, blood cultures remained positive for
C. auris
after five days, and the patient was switched to ibrexafungerp for 17 days. Subsequent blood cultures at Day 3 of ibrexafungerp therapy were negative for
C. auris
, and the patient was considered to have a complete response at the end of therapy. Ibrexafungerp-related adverse events were mild loose stools from days two through four of therapy.
The second patient was a 64-year-old female admitted to the hospital with pneumonia, fever, and hypotension. When
C. auris
was isolated from blood cultures, ibrexafungerp was initiated. A blood culture collected on Day 3 of ibrexafungerp therapy remained positive for
C. auris
and subsequent cultures at Days 9 and 21 were reported negative. The patient improved clinically, received ibrexafungerp for 22 days and was considered a complete response at the end of therapy. No ibrexafungerp-related adverse events were reported.
3.4. Echinocandin Resistance and C. auris
For echinocandins, the primary mechanism of resistance in
C. auris
species comprises the
fks
1 and
fks2 genes, where mutations of the S639F, S639P, and S639Y amino acid sequences were identified as the cause of elevated MICs to echinocandins [14]. Among 350
2 genes, where mutations of the S639F, S639P, and S639Y amino acid sequences were identified as the cause of elevated MICs to echinocandins [9]. Among 350
C. auris
isolates from India, 2% were echinocandin-resistant due to the
fks1 mutation expressing the S639F sequence [29]. A similar finding was reported from Kuwait, where 3 (1.0%) of 314
1 mutation expressing the S639F sequence [53]. A similar finding was reported from Kuwait, where 3 (1.0%) of 314
C. auris
isolates were echinocandin-resistant due to the
fks1 mutation expressing the S639F sequence [30]. Four additional
1 mutation expressing the S639F sequence [54]. Four additional
C. auris
isolates from a total of 106 isolates were resistant to all tested echinocandins (MIC ≥ 4 μg/mL) and contained an S639F mutation in
fks1 [31].
1 [55].
Biagi et al. [32] reported a patient with recurrent candidemia due to
Biagi et al. [56] reported a patient with recurrent candidemia due to
C. auris
that was echinocandin-resistant but azole-sensitive, who expressed the
fks
1 mutation for the S639P sequence. A single
C. auris
isolate was identified in the UK that displayed 5-flucytosine and echinocandin resistance; echinocandin resistance was due to
fks1 mutation for the S639Y sequence [33].
1 mutation for the S639Y sequence [57].
Among
C. auris isolates from India, 8 of 122 with the S639F sequence were echinocandin-resistant with MICs of 4–32 μg/mL; the ibrexafungerp MIC values for these same isolates ranged from 0.25–0.5 μg/mL [20].
isolates from India, 8 of 122 with the S639F sequence were echinocandin-resistant with MICs of 4–32 μg/mL; the ibrexafungerp MIC values for these same isolates ranged from 0.25–0.5 μg/mL [33].