Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 4 by Jessie Wu.
Edible starch-based film was developed for packaging seasoning applied in instant noodles. The edible film can quickly dissolve into hot water so that the seasoning bag can mix in the soup of instant noodles during preparation.
- packaging
- starch film
- edible
- reinforment
1. Introduction
Developing eco-friendly packaging materials is no longer an option, but it has become an urgent necessity since many countries have restricted non-biodegradable materials, particularly for disposable packaging materials. The simplest way to treat the food packaging is to eat them with the foods packaged in. Edible packaging has attracted increasing attention [1][2][3][4], and is mainly based on polysaccharide and protein materials. Starch is the most promising material because of its natural edibility, overwhelming abundance, and annual renewability. Edible starch-based films have been developed and widely used in food and medicine packaging [5][6][7][8], such as applications in candy wrappers and medicine capsules [9][10][11][12].
The bag used for packaging seasonings has been widely used in instant noodles, easy soup, and various pre-prepared ingredients for cooking. It was reported that, on average, everyone ate about 13.6 bags of instant noodles, and the market was more than one myriad bag in 2018 in the world [13]. Traditionally, the films used for flavor bags are bi-orientation polypropylene (BOPP) or polyamide (BOPA) with reasonably excellent mechanical performance and gas barrier properties. Ideally, the packaging film used for the flavor bag would be edible. It could easily dissolve into the hot water during the noodle preparation so that all the treatments before (carefully tearing and pressuring the bag) and after (waste) eating can be omitted, which avoids any environmental issues. An edible starch-based film should be an ideal candidate.
Food and non-food packaging have benefited from starch films’ development [5][10][11][12][14][15]. Improvement of the mechanical properties of starch-based materials is an ongoing challenge due to its poor mechanical performance, particularly tensile strength [16][17][18][19]. Several improvements to the mechanical properties of starch-based materials is an ongoing challenge due to its poor mechanical performance, and tensile strength compositing and blending strategies have been invented to increase these mechanical qualities, such as reinforcing with mineral and natural fillers, or mixing with various decomposable polyesters [20][21][22][23][24][25][26][27][28][29]. However, any additive in edible packaging films is sensitive because of a safety issue or a hazardous risk. All the additives, including plasticizers and reinforcing agents, must be food-grade ingredients.
2. Effect of Various Additives on Performance of Starch-Based Film
The instant noodles were manufactured in less than 50 RH% conditions, and the water content contained in the noodles itself was less than 5%. The relative humidity in the plastic bag or container is about 20–30% RH. Thus, all the performances of the film were evaluated under 20% RH to meet the conditions. Table 1 lists the effect of various additives on starch-based film, including tensile properties, WVTR, and contact angle. The effect of each additive was initially investigated individually, and then optimized based on the results.
Table 1. Effect of various ingredients on the starch film mechanical performances, WVTR, and contact angle.
| Sample Code | Modulus (MPa) | Tensile Str (MPa) | Elongation (%) | WVTR (g/(m2 h)) | Contact Angle (Ɵ) |
|---|---|---|---|---|---|
| Starch Film | 1352 ± 119 a | 45.3 ± 2.9 a | 3.9 ± 0.7 b | 16.2 ± 3.4 a | 82.0 ± 2.9 c |
| C-1 | 1387 ± 102 b | 45.5 ± 4.8 b | 3.7 ± 0.7 a | 16.0 ± 2.8 ab | 83.6 ± 2.7 b |
| C-2 | 1429 ± 118 ab | 47.2 ± 4.7 ab | 3.4 ± 1.1 ab | 15.6 ± 1.9 ab | 85.5 ± 3.3 ab |
| C-3 | 1492 ± 72 a | 48.2 ± 5.2 ab | 3.3 ± 0.8 ab | 15.3 ± 3.5 b | 85.7 ± 3.6 ab |
| C-4 | 1533 ± 126 a | 51.4 ± 5.6 a | 2.6 ± 0.6 b | 16.1 ± 2.7 a | 87.9 ± 4.1 a |
| L-1 | 1382 ± 122 b | 46.8 ± 3.9 b | 3.4 ± 2.1 a | 6.7 ± 0.5 a | 91.4 ± 3.6 a |
| L-2 | 1410 ± 116 ab | 47.7 ± 4.8 ab | 3.1 ± 1.6 ab | 5.7 ± 0.4 b | 105.8 ± 4.2 b |
| L-3 | 1432 ± 104 a | 51.1 ± 5.9 a | 2.7 ± 0.2 b | 5.3 ± 0.6 b | 119.9 ± 3.8 c |
| X-1 | 1242 ± 79 a | 42.6 ± 3.2 a | 8.6 ± 2.2 d | 13.2 ± 0.8 b | 87.7 ± 3.9 a |
| X-2 | 967 ± 74 b | 36.1 ± 3.1 b | 11.3 ± 1.5 c | 14.8 ± 1.3 ab | 94.2 ± 3.4 a |
| X-3 | 818 ± 81 c | 27.0 ± 2.3 c | 19.9 ± 2.1 b | 15.7 ± 1.1 a | 102.4 ± 2.8 b |
| X-4 | 620 ± 66 d | 21.3 ± 1.7 d | 28.4 ± 2.3 a | 15.5 ± 1.6 a | 103.9 ± 2.7 c |
| CX film | 925 ± 73 b | 37 ± 3.6 b | 16.1 ± 2.6 a | 15.6 ± 0.5 a | 91.8 ± 2.7 b |
| CLX film | 965 ± 81 b | 36 ± 2.3 b | 14.2 ± 2.1 a | 5.8 ± 0.4 b | 97.9 ± 2.9 a |
The films were kept at room temperature under 20% RH. The data were analyzed by one way analysis of variance (ANOVA) and marked as
The films were kept at room temperature under 20% RH. The data were analyzed by one way analysis of variance (ANOVA) and marked as
abcd.
.
3. Effect of Cellulose Crystals
The inclusion of the cellulose crystals considerably enhanced the starch film’s strength, as depicted in Table 1. After the crystals were added, both modulus and tensile strength rose. Because hard crystal particles worked as reinforcing agents, this was predicted. The reinforcing process of stiff particles in a matrix is widely understood, and crystals behave similarly. The modulus and tensile strength of the crystals steadily rose as the crystal content increased. On the other hand, the results demonstrate that adding crystals to the films has a minor effect on the elongation at the break. It has to be pointed out that both starch film and the film reinforced by the cellulose crystals are generally very brittle since the elongations are very low, with a value of only about 3–4%. Because of this brittle behavior, the standard dividends (±) of the elongation is higher (>20%).
The starch film WVTP was not affected by the cellulose crystals. It was not expected that a rigid particle could reduce the gas permeability if it has much low gas permeability, since the gap or weak interface between the particle and matrix significantly increases gas permeability. The results indicate that the interface between the starch and cellulose particle is excellent. CA of the starch film improved marginally after adding the cellulose crystals, indicating the moisture sensitivity of the film decreased.
4. Effect of Laver
The modulus elevated when adding more laver, and steadily increased when enhancing laver concentration, indicating that laver improves the films’ stiffness as predicted. The tensile strength rose slightly as well, especially when the laver loading was more than 20%. One theory is that the stiff fibers strengthen the coatings in the laver cell walls. As with how most other fillers behaved, elongations of starch-based films reduced marginally with laver content, and steadily declined when increasing laver content. Similar to the reinforcement by the cellulose crystals, the elongation standard dividends (±) of the starch-based film comprising laver are also reasonably high.
CA increased as laver content increased, implying that the starch-based film’s moisture sensitivity diminished. The findings explain why the laver is moisture resistant. The laver’s moisture resistance is due to its semi-crystalline cellulose and protein [5].
After adding laver to starch-based films, the WVTP declined significantly, and the WVTP decreased even more when the laver content was raised. Creating a channel with some twists in the film matrix that help water vapors go through reduces WVTP when laver is induced [5]. Furthermore, creating the inter-link between protein and starch [30] may lower gas permeability because the tight interlinking between protein and starch has a lower free volume, and allows for less diffusion of moisture. Edible films produced by a combination of whey protein and starch have shown similar results [31][32].
5. Effect of Xylose
The mechanical performance of starch-based materials relies on plasticizer content (water or others). To achieve reasonable flexibility (toughness) under arid conditions (20% RH), different plasticizers were used in this work. The most common plasticizer system used for starch-comprised products is water with glycerol, since glycerol has assertive moisture absorption behavior and is used to maintain the water in the materials. However, the water is still not stable under low RH conditions. A constant concentration of water/glycerol 10/3 was used in this work, and additional plasticizer xylose from food ingredients was added. Xylose is a monosaccharide with a linear structure which has shown remarkably high efficiency in destroying the order structures, and enhancing the movement of polymer chains. As a result, plasticization efficiency improves. [31] Table 1 displays that xylose considerably enhanced the toughness of the starch film, with elongation increasing by up to eight times. With more xylose, the elongation rate increased. However, when the xylose level increased, the modulus and tensile strength of the material declined.
WVTR slightly decreased after the additional xylose. CA was slightly enhanced by xylose, indicating that the hydrophobicity of the starch film marginally increased. The hydroxyl groups in linear xylose are more flexible and face out, which makes sense [33].
6. Morphologies and Interface
6.1. Surface Morphologies
The linkage between cellulose crystals or laver and the starch matrix was investigated using SEM (see Figure 1). It is visible that the pure starch film has a relatively smooth surface (Figure 1A). The cellulose crystals and laver can be seen on the film’s surface after the film’s dried and shrinking matrix. The interface between the starch matrix and cellulose crystalline (B) or laver (C) was homogeneous, and there was no space between the particles and the matrix, which implies that polysaccharide crystals are fully compatible inside the starch matrix. The mechanism may be applied to describe why the films have better mechanical characteristics.
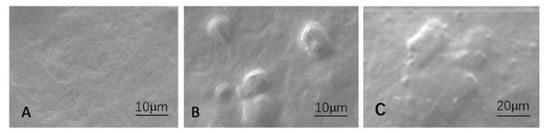

Figure 1. The surface images using SEM. (A) The pure starch film, (B) film containing cellulose crystals, (C) film with the laver.
6.2. Interface
The cross-section images of the films using SEM were used to investigate the linkage between the fillers and the starch matrix (see Figure 2). The pure starch film’s cross-section picture has a smooth surface (a). Even after being sliced, the crystals and laver may still be detected in the starch matrix (b). At higher temperatures, the laver’s cell structure has been disrupted and expanded. These findings were in accordance with the surface morphology investigation (Figure 2), and may be utilized to elaborate mechanical characteristics and moisture barriers. The thin laver also formed a twisted gas channel in the film matrix, which reduced gas permeability.
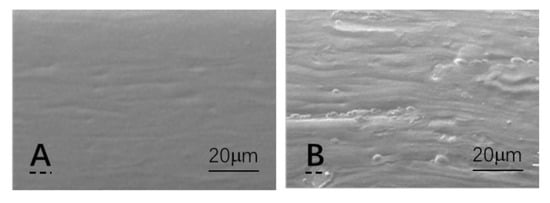

Figure 2. Image of the pure starch film in cross-section using SEM (A). The film containing cellulose crystals and laver (B) (sample CLX).
6.3. Developing Flavor Bag Packaging
The above results helped to develop coating, and extrude the starch-based films and various flavor bag packagings. Figure 3 depicts the images of extruded starch-comprised film and flavor bag packaging without laver (A,B), since some industries require high gas barriers and others do not. The films were developed based on the CX and CLX films, respectively. All the starch-based flavor bags dissolve in hot water (85 °C) within a few seconds.
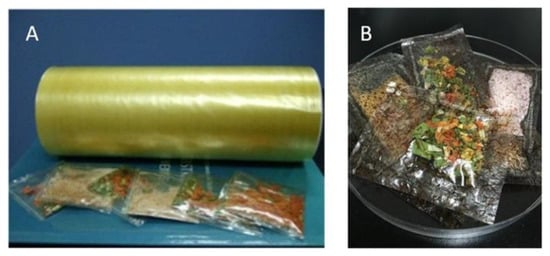

Figure 3. Photos of starch-based film and flavor bag packaging without (A,B) laver.
References
- Patel, P. Edible packaging. ACS Cent. Sci. 2019, 5, 1907–1910.
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845.
- Brody, A.L. Packaging, food. Kirk-Othmer Encycl. Chem. Technol. 2005, 2, 65–66.
- Debeufort, F.; Quezada-Gallo, J.; Voilley, A. Edible films, and coatings: Tomorrow’s packaging: A review. Crit Rev. Food Sci. Nutr. 1998, 38, 299–313.
- Chen, Y.; Yu, L.; Ge, X.; Liu, H.; Ali, A.; Wang, Y.; Chen, L. Preparation and characterization of edible starch film reinforced by laver. Int. J. Biol. Macromol. 2019, 129, 944–951.
- Zhu, J.; Chen, H.; Lu, K.; Liu, H.-S.; Yu, L. Recent progress on starch-based biodegradable materials. Acta Polym. Sin. 2020, 51, 983–995.
- Ali, A.; Xie, F.; Yu, L.; Liu, H.; Meng, L.; Khalid, S.; Chen, L. Preparation and characterization of starch-based composite films reinforced by polysaccharide-based crystals. Compos. Part B 2018, 133, 122–128.
- Ali, A.; Yu, L.; Liu, H.; Khalid, S.; Meng, L.; Chen, L. Preparation and characterization of starch-based composite films reinforced by corn and wheat hulls. J. Appl. Polym. Sci. 2017, 134, 45159.
- Deore, U.V.; Mahajan, H.S. Isolation and characterization of natural polysaccharide from Cassia Obtustifolia seed mucilage as film-forming material for drug delivery. Int. J. Biol. Macromol. 2018, 115, 1071–1078.
- Devi, P.; Bajala, V.; Garg, V.; Mor, S.; Ravindra, K. Heavy metal content in various types of candies and their daily dietary intake by children. Environ. Monit. Assess. 2016, 188, 86.
- Zhang, L.; Wang, Y.; Liu, H.; Yu, L.; Liu, X.; Chen, L.; Zhang, N. Developing hydroxypropyl methylcellulose/hydroxypropyl starch blends for use as capsule materials. Carbohydr. Polym. 2013, 98, 73–79.
- Zhang, L.; Wang, Y.-F.; Liu, H.-S.; Zhang, N.-Z.; Liu, X.-X.; Chen, L.; Yu, L. Development of capsules from natural plant polymers. Acta Polym. Sinica 2013, 1–10.
- China Commercial Information Web. Available online: www.askci.com (accessed on 20 July 2021).
- Yusof, N.L.; Mutalib, N.-A.A.; Nazatul, U.; Nadrah, A.; Aziman, N.; Fouad, H.; Jawaid, M.; Ali, A.; Kian, L.K.; Sain, M. Efficacy of Biopolymer/Starch-Based Antimicrobial Packaging for Chicken Breast Fillets. Foods 2021, 10, 2379.
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and applicability of starch-based films: An eco-friendly approach. Foods 2021, 10, 2181.
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602.
- Nasseri, R.; Mohammadi, N. Starch-based nanocomposites: A comparative performance study of cellulose whiskers and starch nanoparticles. Carbohy Polym. 2014, 106, 432–439.
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626.
- Bras, J.; Dufresne, B. Starch nanoparticles: A review. Biomacromolecules 2010, 11, 1139–1153.
- Gao, C.; Yu, L.; Liu, H.; Chen, L. Development of self-reinforced polymer composites. Prog. Polym. Sci. 2012, 3, 767–780.
- Bodirlau, R.; Teaca, C.-A.; Spiridon, I. Influence of natural fillers on the properties of starch-based biocomposite films. Compos. Part B Eng. 2013, 44, 575–583.
- Wan, Y.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X. Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compo. Sci. Technol. 2009, 69, 1212–1219.
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Nafchi, A.M.; Jokar, M. Preparation, and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7.
- Wilhelm, H.-M.; Sierakowski, M.-R.; Souza, G.; Wypych, F. Starch films reinforced with mineral clay. Carbohydr. Polym. 2003, 52, 101–110.
- Ochoa, T.A.; Almendárez, B.E.G.; Reyes, A.A.; Pastrana, D.M.R.; López, G.F.G.; Belloso, O.M.; Regalado-González, C. Design and characterization of corn starch edible films including beeswax and natural antimicrobials. Food Bioproc. Technol. 2016, 10, 103–114.
- Aldana, D.S.; Andrade-Ochoa, S.; Aguilar, C.N.; Contreras-Esquivel, J.C.; Nevárez-Moorillón, G.V. Antibacterial activity of pectic-based edible films incorporated with Mexican lime essential oil. Food Control 2015, 50, 907–912.
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175.
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1129.
- Duan, Q.; Jiang, T.; Xue, C.; Liu, H.; Liu, F.; Alee, M.; Ali, A.; Chen, L.; Yu, L. Preparation and characterization of starch/enteromorpha/nano-clay hybrid composites. Int. J. Biol. Macromol. 2020, 150, 16–22.
- Sukhija, S.; Singh, S.; Riar, C.S. Analyzing the effect of whey protein concentrate and psyllium husk on various characteristics of biodegradable film from lotus (Nelumbo nucifera) rhizome starch. Food Hydrocolloid. 2016, 60, 128–137.
- Munoz, L.; Aguilera, J.; Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Characterization and microstructure of films made from mucilage of salvia hispanica and whey protein concentrate. J. Food Eng. 2012, 111, 511–518.
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohydr. Polym. 2013, 98, 630–637.
- Alee, M.; Duan, Q.; Chen, Y.; Liu, H.; Ali, A.; Zhu, J.; Jiang, T.; Rahaman, A.; Chen, L.; Yu, L. Engineering, Plasticization Efficiency and Characteristics of Monosaccharides, Disaccharides, and Low-Molecular-Weight Polysaccharides for Starch-Based Materials. ACS Sustain. Chem. Eng. 2021, 9, 11960–11969.
More
