Fatty acid synthase is a multi-enzyme protein that involves in fatty acid synthesis. It's main function is to synthesise palmitate, a 16-carbon long chain saturated fatty acid from acetyl-CoA and malonyl-CoA, in the presence of NADPH.
- lipid synthesis, fatty acid, metabolism
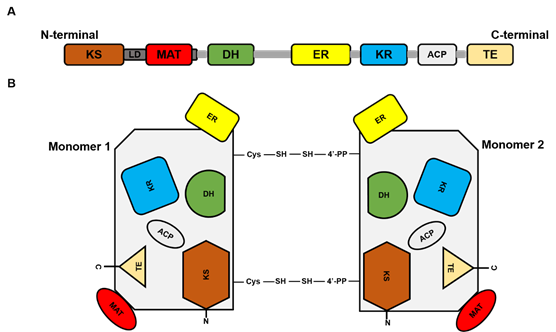
FASN is a large multi-enzyme complex and the monomeric protein size is ~270 kDa. It comprises six separate enzymatic grooves that work together to produce a 16-carbon chain saturated fatty acid (FA) palmitate, from acetyl-coenzyme A (CoA) and malonyl-CoA in the presence of Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) [41]. The FASN monomer (Figure 1) possesses enzymatic activities which include beta-ketoacyl synthase (KS), acetyl/malonyl transacylase (AT/MT), beta-hydroxyacyl dehydratase (DH), enoyl reductase (ER), beta-ketoacyl reductase (KR), acyl carrier protein (ACP), and thioesterase (TE). Although the FASN monomer contains all the necessary enzymes needed for palmitate synthesis, the dimer formation is crucial for its function. The structure of FASN can be further categorized into three major domains where domain I contains KS, AT/MT and DH, domain II contains ER, KR and ACP, and domain III contains TE. About a quarter length of the monomer protein, located between domains I and II, which lacks catalytic activity, is called the interdomain/core region and is identified to be crucial for dimer formation [52].
Figure 1. Fatty acid synthase (FASN) structure. (A) represents the linear sequence organization of FASN monomer. (B). Structural overview of FASN comprising two identical monomers, each including seven catalytic domains: beta-ketoacyl synthase (KS), acetyl/malonyl transacylase (AT/MT), beta-hydroxyacyl dehydratase (DH), enoyl reductase (ER), beta-ketoacyl reductase (KR), acyl carrier protein (ACP), and thioesterase (TE).
FASN expression is critical for early embryo development, in which FASN knockout (KO) embryos fail to survive before implantation and the number of FASN heterozygous pups is 70% lower than predicted by Mendelian Inheritance, which indicate partial haploid insufficiency [63]. Furthermore, FASN expression is shown to participate in the proper development of the fetal lung and the normal functionality of the adult lung. There is ample evidence demonstrating that the fetal lung is capable of de novo FA synthesis and that FASN is required for surfactant production of alveolar epithelial cells [74]. After early development, FASN remains relatively quiescent in most tissues, however the reason why this is so still remains elusive. A plausible explanation is that non-actively proliferating tissues can meet the FAs’ demand from the diet to fulfil their physiological FA requirements. Nonetheless, a strong FASN expression has been reported in the lung, breast, liver, adipose and brain [85].
Deletion of FASN in alveolar type II epithelial cells is found to disrupt surfactant lipid composition and exacerbate injury response to bleomycin-induced fibrosis [96]. The mature mammary gland is a unique lipid metabolizing tissue where, in resting-state, it does not require fatty acid synthesis but strongly induces FASN during pregnancy and lactation [107]. De novo FA synthesis in the mammary gland is responsible for producing short and medium chain FAs in milk, which account for ~15–40% of total FA content [11,128,9]. Mammary gland-specific FASN KO mice are shown to suffer from growth reduction in mammary epithelial cells, alteration of the FAs profile in milk from lactating mothers, and also improper development of the functional lactating mammary gland [130].
FASN is considered as a housekeeping protein in the liver under normal physiological conditions where it controls the hepatic triglyceride mechanism. When carbohydrates are abundant, glucose are converted to FAs with the help of FASN. Excess FAs are then assembled into triglycerides and stored in the form of lipid droplets or secreted as very low-density lipoproteins [114,15,12]. During fasting or under glucose-depleted conditions, lipid droplets undergo lipolysis or catabolism and fatty acid oxidation to produce ketone bodies which are then used as fuel [163]. In the brain, FASN is essential to maintain proper development and control lipid metabolism in neural stem cells. Neural stem cells are responsible, not only for early brain development, but also remain active for an entire life to ensure proper brain function. They divide and generate new nerve cells to enable the brain to adapt to new arrangements. Furthermore, disruption of lipid metabolism in neural stem cells, through the expression of a non-functional FASN, results in learning and memory deficits in humans and mice [174].
There is a clear difference in lipid metabolism between normal and tumor cells. Normal cells derive FAs exogenously while tumor cells derive FAs both exogenously and by de novo synthesis through FASN. Tumors or precursor lesions undergo excessive de novo FA synthesis irrespective of circulating lipid levels. In contrast to normal cells, almost all triacylglycerol FAs in tumor cells are derived from de novo synthesis [6215]. Though de novo synthesis of FA and glycolysis in cancer are closely associated with elevated lipogenic and glycolytic enzymes activities [6316], lipogenesis in cancer did not attract much interest among cancer biologists until the mid-1990s. Renewed interests in lipid metabolism started after the discovery of FASN, formerly known as oncogenic antigen-519, in breast cancer in 1994 [6417]. Eukaryotes harbor two distinct FA synthesis systems [6518]. FASN exists exclusively in the cellular cytoplasmic compartment and is referred to as the type I fatty acid synthesis system while the type II fatty acid synthesis system is present in mitochondria and termed mitochondrial FAS. Both systems produce two distinct products.
Mitochondrial fatty acid synthesis is functionally different from cytoplasmic fatty acid synthesis as it does not contribute significantly to cellular triglyceride storage or phospholipids. Furthermore, products from mitochondrial FAS cannot be substituted by delivery of FAs from extra-mitochondrial origin. The main product of mitochondrial FAS is lipoic acid, which is an important lipid co-factor for multiple mitochondrial dehydrogenases and is crucial for optimal mitochondrial function [6619]. Up until now, little is known on mitochondrial FAS and its association with carcinogenesis. Extensive studies have focused on the cytoplasmic FA synthesis system, which is a type I FA system or FASN, and have sparked huge interests to investigate the roles of FASN in cancer. Like other proteins, FASN’s function is greatly affected by its localization, post-translational modification and protein–protein association. Although FASN has been frequently described as a cytoplasmic protein, it can also be found to localize in other intracellular compartments such as the nucleus [20] and membrane of peroxisomes [21].
References:
1. Ventura, R.; Mordec, K.; Waszczuk, J.; Wang, Z.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G.; Heuer, T.S. Inhibition of de novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. EBioMedicine 2015, 2, 808–824.
2. Chirala, S.S.; Wakil, S.J. Structure and function of animal fatty acid synthase. Lipids 2004, 39, 1045–1053.
3. Chirala, S.S.; Chang, H.; Matzuk, M.; Abu-Elheiga, L.; Mao, J.; Mahon, K.; Finegold, M.; Wakil, S.J. Fatty acid synthesis is essential in embryonic development: Fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc. Natl. Acad. Sci. USA 2003, 100, 6358–6363.
4. Rooney, S.A. Fatty acid biosynthesis in developing fetal lung. Am. J. Physiol. Cell. Mol. Physiol. 1989, 257], L195–L201.
5. Semenkovich, C.F.; Coleman, T.; Fiedorek, F.T. Human fatty acid synthase mRNA: Tissue distribution, genetic mapping, and kinetics of decay after glucose deprivation. J. Lipid Res. 1995, 36, 1507–1521.
6. Chung, K.-P.; Hsu, C.-L.; Fan, L.-C.; Huang, Z.; Bhatia, D.; Chen, Y.-J.; Hisata, S.; Cho, S.J.; Nakahira, K.; Imamura, M.; et al. Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis. Nat. Commun. 2019, 10, 3390.
7. Rudolph, M.C.; McManaman, J.L.; Phang, T.; Russell, T.; Kominsky, U.J.; Serkova, N.J.; Stein, T.; Anderson, S.M.; Neville, M.C. Metabolic regulation in the lactating mammary gland: A lipid synthesizing machine. Physiol. Genom. 2007, 28, 323–336.
8. Smith, S.; Dils, R. Factors affecting the chain length of fatty acids synthesised by lactating-rabbit mammary glands. Biochim. Biophys. Acta 1966, 116, 23–40.
9. Smith, S.; Gagné, H.T.; Pitelka, D.R.; Abraham, S. The effect of dietary fat on lipogenesis in mammary gland and liver fromem lactating and virgin mice. Biochem. J. 1969, 115, 807–815.
10. Suburane of perou, J.; Shi, L.; Wu, J.; Wang, S.; Samuel, M.; Thomas, M.J.; Kock, N.D.; Yang, G.; Kridel, S.; Chen, Y.Q. Fatty acid synthase is required for mammary gland development and milk production during lactation. Am. J. Physiol. Metab. 2014, 306, E1132–E1143.
11. Hudgins, L.C.; Hellerstein, M.; Seidman, C.; Neese, R.; Diakun, J.; Hirsch, J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Investig. 1996, 97, 2081–2091.
12. Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753.
13. Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahlisomes, W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498.
14. Bowers, M.; Liang, T.; Gonzalez-Bohorquez, D.; Zocher, S.; Jaeger, B.N.; Kovacs, W.J.; Röhrl, C.; Cramb, K.M.; Winterer, J.; Kruse, M.; et al. FASN-Dependent Lipid Metabolism Links Neurogenic Stem/Progenitor Cell Activity to Learning and Memory Deficits. Cell Stem Cell 2020.
15. Medes, G.; Thomas, A.; Weinhouse, S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953, 13, 27–29.
16. Szutowicz, A.; Kwiatkowski, J.; Angielski, S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br. J. Cancer 1979, 39, 681–687.
17. Kuhajda, F.P.; Jenner, K.; Wood, F.D.; Hennigar, R.A.; Jacobs, [L.B.; Dick, J.D.; Pasternack, G.R. Fatty acid synthesis: A potential selective target for antineoplastic therapy. Proc. Natl. Acad. Sci. USA 1994, 91, 6379–6383.
18. Kastaniotis, A.J.; Autio, K.J.; Kerätär, J.M.; Monteuuis, G.; Mäkelä, A.M.; Nair, R.R.; Pietikäinen, L.P.; Shvetsova, A.; Chen, Z.; Hiltunen, K. Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 39–48.
19. Hiltunen, K.; Autio, K.J.; Schonauer, M.S.; Kursu, V.S.; Dieckmann, C.L.; Kastaniotis, A.J. Mitochondrial fatty acid synthesis and respiration. Biochim. Biophys. Acta 2010, 1797, 1195–1202.
20. Madigan, A.A.; Rycyna, K.J.; Parwani, A.V.; Datiri, Y.J.; Basudan, A.M.; Sobek, K.M.; Cummings, J.L.; Basse, P.H.; Bacich, D.J.; O’Keefe, D.S. Novel Nuclear Localization of Fatty Acid Synthase Correlates with Prostate Cancer Aggressiveness. Am. J. Pathol. 2014, 184, 2156–2162.
21. Hillebrand, M.; Gersting, S.W.; Lotz-Havla, A.S.; Schäfer, A.; Rosewich, H.; Valerius, O.; Muntau, A.C.; Gärtner, J. Identification of a New Fatty Acid Synthesis-Transport Machinery at the Peroxisomal Membrane. J. Boil. Chem. 2011, 28]7, 210–221.