Biomarkers can be used for diagnosis, prognosis, and prediction in targeted therapy. The estrogen receptor α (ERα) and human epidermal growth factor receptor 2 (HER2) are standard biomarkers used in breast cancer for guiding disease treatment. The androgen receptor (AR), a nuclear hormone receptor, contributes to the development and progression of prostate tumors and other cancers. With increasing evidence to support that AR plays an essential role in breast cancer, AR has been considered a useful biomarker in breast cancer, depending on the context of breast cancer sub-types. The existing survival analyses suggest that AR acts as a tumor suppressor in ER + ve breast cancers, serving as a favorable prognostic marker. However, AR functions as a tumor promoter in ER-ve breast cancers, including HER2 + ve and triple-negative (TNBC) breast cancers, serving as a poor prognostic factor. AR has also been shown to be predictive of the potential of response to adjuvant hormonal therapy in ER + ve breast cancers and to neoadjuvant chemotherapy in TNBC.
All contents are adapted from You, C.-P.; Leung, M.-H.; Tsang, W.-C.; Khoo, U.-S.; Tsoi, H. Androgen Receptor as an Emerging Feasible Biomarker for Breast Cancer. Biomolecules 2022, 12, 72. https://doi.org/10.3390/biom12010072
- breast cancer
- androgen receptor
- biomarker
- targeted therapy
1. What Are Cancer Biomarkers
2. AR as a Biomarker in Breast Cancers
Breast cancer is the most common malignancy in the female population. According to the molecular expression profiles, breast cancers can be classified into five biologically distinct sub-types: luminal A, luminal B, HER2-enriched (HER2 + ve), basal-like, and normal-like [6][39]. Luminal A and normal-like tumors were characterized by hormone-receptor-positive (ER-positive and/or PR-positive) with HER2-ve and low Ki-67. Luminal B tumors were defined by hormone-receptor-positive with either HER2 + ve or HER2-ve and high Ki-67. The basal-like sub-type lacks ERα, PR, and HER2; it was therefore regarded as triple-negative breast cancer (TNBC). The luminal A sub-type has the best treatment outcome, while the basal-like sub-type has the worst in the clinic [7][8][40,41]. The subtyping of breast cancers largely determines the subsequent treatment of the patients. Surprisingly, AR is also prevalent in up to 90 % of all breast cancers [9][42]. Based on the experience of treating prostate cancer, the possible involvement of AR in the pathogenesis of breast cancer has attracted consideration from investigators. The outcome of clinical studies on AR over the past decades in different sub-types of breast cancers, as documented in Table 1, remain controversial as to whether the AR is a good or poor prognostic factor in breast cancers. Most of the earlier studies were solely focused on the AR molecular profile while ignoring the biological interactions between AR and intrinsic molecular differences in the tumors. Since breast cancers are molecularly heterogeneous, and the growth of the tumor results from the contribution of various molecules, the role of AR in breast cancers needs to be discussed separately for the different sub-types (Figure 2).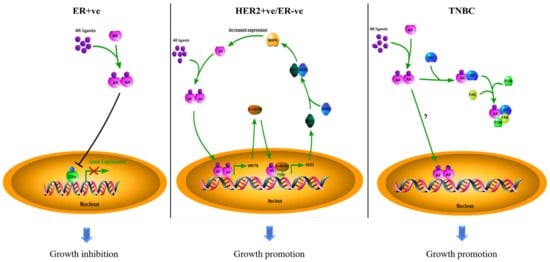
| Types | AR Status (Cut-Off Used to Define AR + ve) | Case No. | Indicator of Clinical Outcomes 1 | Hazard Ratio (HR) | 95% Confidence Interval (CI) | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| ER + ve | Positive (≥10% nuclear-stained) | 470 | DFS | 0.654 | 0.429–0.997 | 0.049 | [10][44] |
| Negative (<10% nuclear-stained) | 202 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 1024 | OS | 0.68 | 0.52–0.88 | - | [11][45] | |
| Negative (<1% nuclear-stained) | 140 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 2833 | BCM | 0.53 | 0.41 –0.69 | < 0.001 | [12][46] | |
| Negative (<1% nuclear-stained) | 470 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 609 | DSS | 0.259 | 0.139–0.482 | 0.000 | [13][47] | |
| Negative (<1% nuclear-stained) | 250 | 1 | - | - | |||
| High (mRNA Z-score) | 145 | DRFS | - | - | 0.008 | [14][48] | |
| Low (mRNA Z-score) | 144 | - | - | - | |||
| Positive (N/A) | - | DFS | 0.40 | 0.31–0.52 | < 0.001 | [15][54] | |
| Negative (N/A) | - | 1 | - | - | |||
| Positive (≥10% nuclear-stained) | 909 | OS | 0.71 | 0.53–0.95 | 0.022 | [16][55] | |
| Negative (<10% nuclear-stained) | 162 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 461 | DFS | 0.606 | 0.388–0.944 | 0.027 | [17][56] | |
| Negative (<1% nuclear-stained) | 337 | 1 | - | - | |||
| HER2 + ve/ ER-ve |
Positive (≥10% nuclear-stained) | 49 | OS | - | - | 0.074 | [10][44] |
| Negative (<10% nuclear-stained) | 42 | - | - | - | |||
| High (mRNA level) | 35 | DFS | 1.46 | 1.03–2.06 | 0.03 | [18][57] | |
| Low (mRNA level) | 49 | 1 | - | - | |||
| TNBC | Positive (≥1% nuclear-stained) | 78 | OS | 1.83 | 1.11–3.01 | 0.02 | [11][45] |
| Negative (<1% nuclear-stained) | 133 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 261 | OS | 2.159 | 1.224–3.808 | 0.008 | [19][58] | |
| Negative (<1% nuclear-stained) | 231 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 23 | DFS | 5.26 | 1.39–19.86 | 0.014 | [20][59] | |
| Negative (<1% nuclear-stained) | 38 | 1 | - | - | |||
| Positive (≥1% nuclear-stained) | 78 | DDFS | 1.82 | 1.10–3.02 | 0.020 | [21][60] | |
| Negative (<1% nuclear-stained) | 185 | 1 | - | - |
