Diabetes mellitus, especially type 2 (T2DM), is a major public health problem globally. DM is characterized by high levels of glycemia and insulinemia due to impaired insulin secretion and insulin sensitivity of the cells, known as insulin resistance. T2DM causes multiple and severe complications such as nephropathy, neuropathy, and retinopathy causing cell oxidative damages in different internal tissues, particularly the pancreas, heart, adipose tissue, liver, and kidneys. Isorhamnetin, a plant flavonoid, has long been studied for its potential anti-diabetic effects. Isorhamnetin is a monomethoxyflavone or an O-methylated flavonol from the class of flavonoids. It is quercetin in which a methoxy group replaces the hydroxy group at position 3’. Some isorhamnetin derivatives are present in nature, such as isorhamnetin 3-O-β-d-glucopyranoside, isorhamnetin 3-O-neohesperidoside, and isorhamnetin 3-O-rutinoside from Calendula officinalis L.. Isorhamnetin presents significant biological properties such as antioxidant, anticancer, antimicrobial, antiviral, anti-inflammatory and anti-diabetic effects.
- isorhamnetin
- quercetin
- biological activities
- diabetes
- molecular pathways
1. General Overview of Biological Activities of Isorhamnetin
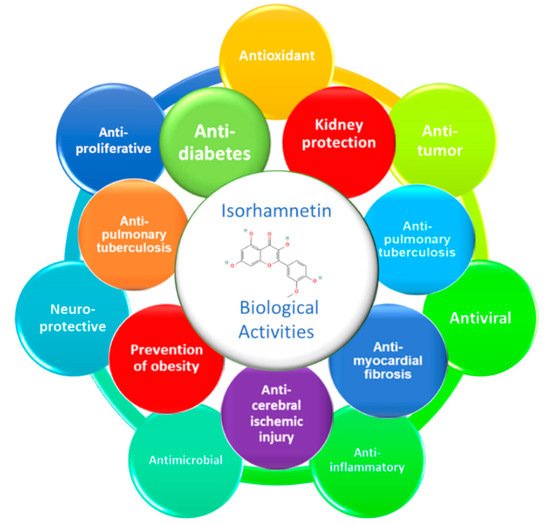
2. Anti-Diabetic Effect of Isorhamnetin
2.1. General Overview on Diabetes and Its Link with Metabolic Syndrome
2.2. Effect of Isorhamnetin on Associated Metabolic Pathways
| Plant Sources | Extraction Methods | Action Modes | References |
|---|---|---|---|
| Vaccinium vitis-idaea | Fractionation guided 80% ethanol > ethyl acetate > water |
Enhances muscle cell glucose uptake | [48][98] |
| Nitraria retusa | Maceration in water-ethanol solution | Modulation of lipogenesis–lipolysis balance | [49][17] |
| Nitraria retusa | Maceration in water-ethanol solution | 3T3-L1 preadipocyte differentiation regulation | [50][18] |
| Corchorus olitorius | Soxhlet extraction with methanol, the residue from the above soluble extraction was hydrolyzed directly with 200 mL of 4 M NaOH solution. Then, the mixture was adjusted to pH 2 with concentrated HCl and the bound phytochemicals were extracted with ethyl acetate. | - α-Amylase inhibition - α-Glucosidase inhibition - Angiotensin I converting enzyme (ACE) inhibition - Degradation of deoxyribose |
[44][95] |
| Salicornia herbacea | Fractionation guided ethanol under reflux > ethyl acetate ethyl acetate fraction was eluted on a silica gel chromatography by gradient elution with chloroform and methanol |
- α-Glucosidase - Regulating the expression of ERK, PI3K, AKT, IRS-2, and PDX-1 |
[45][96] |
| Eruca sativa | Extraction with ethanol by the accelerated solvent extractor, at 50 °C, 1500 psi for 20 min | Activation of PPAR-α and suppression of inflammatory cytokines | [51][28] |
| Oenanthe javanica | Isorhamnetin was isolated from the aerial part of O. javanica and its purity was higher than 95.0% | Attenuation of fibrosis in rat hepatic stellate cells via inhibition of ERK signaling pathway | [52][99] |
