Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Lida Fuentes-Viveros.
The peumo (Cryptocarya alba) is a native fruit from central Chile that belongs to the Lauraceae family. To characterize the development and the potential health benefits of this edible fruit, quality and physiological parameters, along with antioxidant capacity, were evaluated during three clearly defined developmental stages of the fruit in two seasons.
- functional food
- antioxidants
- peumo
1. Background
The peumo [Cryptocarya alba (Molina) Looser] is a Chilean Lauraceae tree with an endemic spread from Maule to the Araucania Regions. It is considered a threatened species in some areas of Chile, mainly due to overexploitation and habitat destruction [1]. Concerning its ecological importance, peumo is one of the representative species of the sclerophyllous forest of the central zone of Chile, including boldo (Peumus boldus), quillay (Quillaja saponaria), and hawthorn (Acacia caven) [2]. All sclerophyllous species are frequently used in low water consumption gardens. Likewise, quillay and boldo are species with interesting pharmacological and industrial applications of their compounds, being an important source of saponins [3] and boldine [4], respectively.
Despite no agro-industrial use, peumo leaves have been used in traditional medicine like infusion or ointment [2,5][2][5]. On the other hand, this tree has beautiful and pink-colored berries (Figure 1), called peumos, collected and consumed by the Mapuche Amerindians, principally as a cold infusion, since pre-Colombian times. This fruit is composed of edible and pink skin, with intense flavor and aroma at maturity, and a large seed like a nut; these characteristics have allowed its gastronomic use in recent years [2].
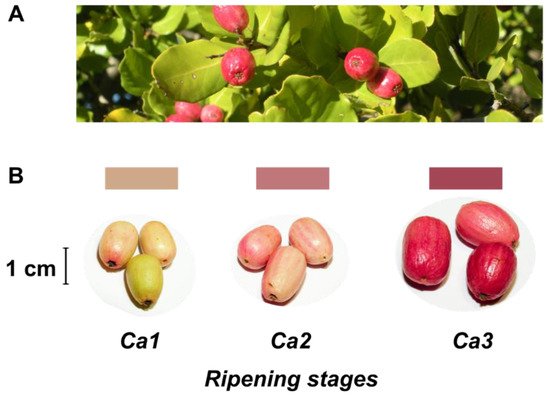
Figure 1. Leaves and fruits of peumo tree. (A) Leaves and fruits of peumo [Cryptocarya alba (Molina) Looser]; (B) Three different development stages of peumo fruit. Photography credit: Lida Fuentes.
The essential oil of this species was reported to be composed mainly of 1-terpinen-4-ol and p-cimol [6], while the cryptofolione derivative has been the only compound isolated from the edible fruits [7]. Domínguez and Martínez (2002) [8] reported that the peumo fruit skin has many polyphenols, but it is unclear if this potential is linked to a particular genotype. Simirgiotis (2013) [9] reported a high antioxidant capacity (9.12 ± 0.01 mg·mL−1), determined by DPPH assay in ripe fruit and flavonoid glycosides, phenolic acids, anthocyanins, and flavonoid aglycons as the major phenolic compounds in fruit and aerial parts of peumo extracts.
The antioxidant capacity of native Chilean fruits like murtilla (Ugni molinae Turcz.) (10,770 ± 453), maqui (Aristotelia chilensis (Molina) Stuntz) (19,850 ± 966), and calafate (Berberis sp.) (25,662 ± 3322), determined by oxygen radical absorbance capacity (ORAC: μmol TE/100 g fresh weight), has been described as higher than commercial berries such as raspberries (6903 ± 1019), blueberries (8869 ± 334), and blackberries (9043 ± 1253) [10]. Furthermore, this antioxidant capacity has been associated with a high functional potential [10,11,12][10][11][12]. Likewise, water-soluble extracts of maqui berry have been reported to prevent the oxidation of copper-induced low-density lipoprotein (LDL), adipogenesis, and inflammatory actions, and to protect human endothelial cell cultures [12,13,14,15,16][12][13][14][15][16].
The quality, physiological parameters, and potential health benefits of many attractive native fruits could be affected by inadequate handling postharvest. Therefore, knowledge about the fruit’s physiological and physicochemical parameters before studying the healthy potential of native fruit is relevant.
2. Characterization of Quality and Physiological Parameters during Fruit Development of the Peumo Fruit
The present study classified three developmental stages of peumo (C. alba) fruits (Figure 1). A constant boost in fruit fresh and dry weights was registered according to ripening during the first harvest season, and non-significant differences between Ca2 and Ca3 stages were observed during the second season (Table 1). The water activity displays non-significant differences during ripening, with a similar decrease throughout both seasons. The fruit length increased during both seasons, while the diameter only increased for the 2017 season; despite the above, the fruits have a thin shape in all analyzed stages and both seasons (Figure 1, Table 1). The fruit firmness displays a constant reduction during ripening in both seasons, with firmness in ripe fruit near 5 N (Table 1).
Table 1. Changes in physicochemical and physiological parameters during fruit development of peumo fruit for the 2017 and 2018 harvest seasons.
| Developmental Stages | |||||||
|---|---|---|---|---|---|---|---|
3. Antioxidant Capacity, Total Polyphenol, and Flavonoid Content during Fruit Development of the Peumo Fruit
Determinations of total antioxidant capacity by four different methods (FRAP, TEAC, DPPH, and ORAC assays) indicated that the increase in antioxidant capacity, according to the ripening progress, displayed significant differences between the Ca1 and Ca3 stages. This trend was similar to those observed for total polyphenols content (TPC) and total flavonoids content (TFC) (Table 2). However, no differences were observed in the ORAC method for the second season. The TPC in ripe peumo was near 17 mg GA/g FW, and TFC was near 9 mg QE/g FW in both seasons; these values were higher than those determined for ripe blueberries in the 2018 season (TPC:2.75 mg GA/g FW and TFC: 2.12 mg QE/g FW).
Table 2. Total polyphenol content (TPC), total flavonoid content (TFC), antioxidant capacity by FRAP, TEAC, DPPH, and ORAC methods, during the development of peumo fruits for the 2017 and 2018 harvest seasons. GAE, gallic acid equivalent; QE, quercetin equivalent; TE, trolox equivalent.
| Development Stage | TPC [mgGAE/gFW] | TFC [mgQE/gFW] | FRAP [μmol FeSO | 4 | /gFW] | TEAC [mmol TE/gFW] | DPPH [IC | 50 | μg/mL] | ORAC [mmol TE/gFW] |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Harvest Season 2017 | Harvest Season 2018 | ||||||||||||||||||
| Season 2017 | Ca1 | Ca2 | Ca3 | Ca1 | Ca2 | Ca3 | ||||||||||||||
| 0.032 ± 0.000 | ||||||||||||||||||||
Data correspond to the means ±SE of four replicates of fruit mix for each stage and season. Different letters point to significant differences between developmental stages in each parameter (p ≤ 0.05). n.a., not analyzed.
The principal component analysis (PCA) of antioxidant capacity for the 2017 (Figure A1A) and 2018 (Figure A1B) harvest seasons describes a similar behavior for FRAP, TFC, and TEAC analysis. However, DPPH and TPC have different behavior for each season, with a high correlation with the other antioxidant variables in the 2017 season and a mainly orthogonal location (not correlated) for 2018 season. The correlation between antioxidant analysis for the 2017 (Table A1) harvest season describes a significant correlation, where FRAP, TEAC, and TFC are the most correlated. Nevertheless, we have a different correlation for the 2018 (Table A2) season, where TPC and DPPH describe not correlated behavior. However, FRAP, TEAC, and TFC still present a positive correlation between them in the season.
4. Composition of Peumo Fruit Extract
The chemical composition of peumo fruit extract was determined by non-target analysis using U-HPLC/MS LTQ in both positive and negative modes (Figure 2). Identified compounds (Table 3) included many flavonoids, alkaloids, and lignins. The main part of flavonoids was represented by quercetin and its derivatives and metabolites, followed by proanthocyanidins (namely procyanidins), phenols (catechin and epicatechin) and polyphenols (chlorogenic acid and its analogue, 4-caffeoylquinic acid), flavones (luteolin 7-O-glucuronide, sexangularetin), and other flavonoids. Lignans were represented by 4-O-methylcedrusin and (+)-lariciresinol. Alkaloids were represented by cryprochine and its stereoisomer. The high content of flavonoids could be responsible for the antioxidant and anti-inflammatory activity of peumo extract. Also, the ORAC value of peumo extract (500 mg/mL) was 0.637 ± 0.061 mmol/g DW (Table 3).
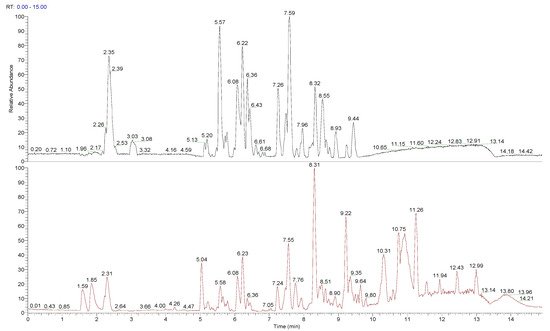
Figure 2. Total ion chromatogram (TIC) for peumo, presented in negative and positive modes.
Table 3. Identification of compounds and antioxidant capacity from the methanol extract of peumo fruits, by LC-MS and MS/MS data. The principal peaks were individually analyzed, and the potential molecules were identified. Also, total polyphenol content (TPC), total flavonoid content (TFC), and antioxidant capacity by ORAC methods were determined. GAE, gallic acid equivalent; QE, quercetin equivalent; TE, trolox equivalent.
| RT (min) | [M + X] | + | (m/z) |
[M − X] | − | (m/z) |
[M] (m/z) |
Fragments | MF | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene production | nd* | nd* | nd* | nd* | nd* | nd* | ||||||||||||||||||||||||||||||||
| Tentative Compound | ||||||||||||||||||||||||||||||||||||||
| Ca1 | 11.19 ± 0.7.4 | b | 7.34 ± 0.27 | c | 28.62 ± 0.95 | b | 4.34 ± 0.34 | |||||||||||||||||||||||||||||||
| 2.35 | 343.1236 [M + H] | + | 341.1112 [M − H] | − | 683.2303 [2M − H] | c | − | 6.84 ± 0.17 | b | 342 | −89.0227, 101.0226, 119.0330, 143.0329, 161.0433, n.a. |
179.0537 | C | 12 | H | 22 | O | 11 | Sucrose | |||||||||||||||||||
| Oxygen consumption (mg Kg | −1 | h | −1 | ) | 6.73 ± 0.00 a | 5.85 ± 0.04 b | 1.88 ± 0.04 c | 6.98 ± 0.01 a | 5.99 ± 0.02 b | 2,07 ± 0.02 c | ||||||||||||||||||||||||||||
| Ca2 | 13.70 ± 1.03 | b | 8.52 ± 0.33 | b | 35.96 ± 0.36 | a | 7.23 ± 0.32 | b | 6.89 ± 0.54 | b | n.a. | |||||||||||||||||||||||||||
| 5.04 | 316.2121 [M + H] | + | 315 | 102.0916, 123.1169, 184.1695, 255.1590 |
C | 19 | H | 25 | NO | 3 | CO | 2 | production (mg Kg | −1 | h | −1 | ) | Ca3 | 17.87 ± 1.57 | a | 9.21 ± 0.28 | a | 38.34 ± 0.33 | a | 8.09 ± 0.22 | a | 8.72 ± 0.14 | a | n.a. | |||||||||
| 5.13 | 867.2132 [M + H] | + | 865.2036 [M − H] | − | 866 | −287.0587 −407.0808 −451.1076 −577.1406 −695.1471 713.1580 −739.1739 −847.0959 |
C | 45 | H | 38 | O | 18 | Procyanidin C | 1 | Season 2018 | |||||||||||||||||||||||
| 5.20 | 579.1500 [M + H] | + | 1155.2760 [2M + H] | + | 577.1387 [M − H] | − | 1153.2665 [2M − H]− |
578 | −289.0744 −407.0809 −425.0918 −451.1076 |
Ca1 | 12.85 ± 1.16 | c | 6.98 ± 0.21 | c | 29.49 ± 2.36 | b | 5.02 ± 0.39 | b | 7.69 ± 0.90 | b | 0.208 ± 0.010 | a | ||||||||||||||||
| Cryprochine | Isocryprochine | 8.53 ± 0.00 a | 7.49 ± 0.04 b | 3.29 ± 0.04 c | 6.86 ± 0.04 a | 6.43 ± 0.02 b | 5.30 ± 0.51 c | |||||||||||||||||||||||||||||||
| Firmness (N) | 7.54 ± 0.20 a | 5.99 ± 0.23 b | 4.97 ± 0.15 c | 7.93 ± 0.76 a | 6.04 ± 1.14 b | 5.03 ± 0.65 c | ||||||||||||||||||||||||||||||||
| C | 30 | H | 26 | O | 12 | Procyanidin B | 1 | Procyanidin B | 2 | pH | ||||||||||||||||||||||||||||
| 5.57 | 5.66 ± 0.00 c | 355.1025 5.75 ± 0.00 b |
[M + H] | + | 5.90 ± 0.01 a | 377.0845 [M + Na] | + | 353.0900 [M − H] | − | 707.1879 [2M − H] | − | 3546.12 ± 0.05 a | 6.26 ± 0.02 a | 6.26 ± 0.03 a | ||||||||||||||||||||||||
| 135.0458 | 179.0362 | C | 16 | H | 18 | O | 9 | 4-Caffeoylquinic acid | TA (%) | nd* | nd* | nd* | nd* | nd* | Ca2 | 15.15 ± 0.71 | and* | |||||||||||||||||||||
| 579.1500 [M + H] | + | 8.46 ± 0.39 | b | 35.94 ± 1.23 | 577.1386 [M − H]a |
− | 7.12 ± 0.18 | a | 7.09 ± 0.19 | 578 | C | 30 | H | 26 | O | 12 | a | 0.199 ± 0.002 | a | SSC (°Brix) | ||||||||||||||||||
| Procyanidin B | 1 | Procyanidin B | 2 | 24.72 ± 2.18 b | 32.50 ± 1.78 a | 38.06 ± 4.16 a | 30.00 ± 2.50 a | 25.83 ± 1.44 a | 29.17 ± 1.44 a | |||||||||||||||||||||||||||||
| Ca3 | 17.61 ± 0.60 | a | 9.44 ± 0.18 | a | ||||||||||||||||||||||||||||||||||
| 867.2131 [M + H] | 37.08 ± 0.75 | + | 865.2020 [M − H] | − | 866 | a | 7.91 ± 0.30 | C | 45 | H | a | 38 | 8.35 ± 0.53 | a | O | 0.188 ± 0.002 | a | |||||||||||||||||||||
| 18 | Procyanidin C1 | Length (cm) | 1.25 ± 0,02 b | 1.34 ± 0.02 b | 1.59 ± 0.05 a | 1.76 ± 0.10 b | 2.08 ± 0.04 a | 2.10 ± 0.04 a | ||||||||||||||||||||||||||||||
| Blueberry | 2.75 ± 0.2 | |||||||||||||||||||||||||||||||||||||
| 6.08 | 355.1027 [M + H] | + | 377.0846 | 2.12 ± 0.44 | [M + Na] | + | 4.95 ± 0.28 | 1.25 ± 0.30 | 731.1894 [2M + Na] | + | 353.0903 [M − H] | − | 707.1885 [2M − H] | − | 11.36 ± 0.96 | 354 | 191.0575 | C | 16 | H | 18 | O | 9 | Chlorogenic acid | Diameter (cm) | 0.91 ± 0.02 c | ||||||||||||
| 6.22 | 291.0863 [M + H] | + | 0.97 ± 0.01 b | 1.08 ± 0.02 a |
1.21 ± 0.04 a | 313.0680 [M + Na] | + | 289.0737 [M − H] | − | 353.0900 1.25 ± 0.02 a |
579.1549 [2M − H] | − | 290 | −179.0357, 1.22 ± 0.01 a | ||||||||||||||||||||||||
| 205.0516 | 245.0811 | C | 15 | H | 14 | L/D | 1.38 ± 0.02 b | 1.38 ± 0.02 b | 1.47 ± 0.03 a | 1.45 ± 0.05 b | 1.66 ± 0.02 a | 1.73 ± 0.05 a | ||||||||||||||||||||||||||
| FW (g) | 0.58 ± 0.03 c | [M + H] | + | 0.79 ± 0.02 b | 1.19 ± 0.05 a | 1.75 ± 0.17 a | 2.08 ± 0.08 a | 731.1821 [2M + Na] | + | 1.99 ± 0.05 a | ||||||||||||||||||||||||||||
| 353.0901 | [M − H] | − | 707.1879 [2M − H] | − | 354 | −191.0568 | C | 16 | H | 18 | O | 9 | Analogue of chlorogenic acid 4-Caffeoylquinic acid |
DW (g) | 0.32 ± 0.02 c | 0.46 ± 0.01 b | 0.69 ± 0.03 a | 1.07 ± 0.11 a | 1.26 ± 0.06 a | 1.13 ± 0.03 a | ||||||||||||||||||
| Humidity | ||||||||||||||||||||||||||||||||||||||
| 7.26 | 465.1031 | wet basis | 43.10 ± 0.43 a | 41.77 ± 0.54 b | 40.34 ± 0.47 b | 42.29 ± 0.46 a | 41.35 ± 1.20 a | 40.70 ± 0.63 b | ||||||||||||||||||||||||||||||
| [M + H] | + | 487.0849 [M + Na] | + | Humidity | dry basis | 75.76 ± 0.29 a | 71.74 ± 0.05 b | 67.61 ± 0.07 b | 73.27 ± 0.16 a | 70.49 ± 0.55 a | 66.86 ± 0.12 b | |||||||||||||||||||||||||||
| Water activity | 0.81 ± 0.01 a | 0.78 ± 0.02 a | 0.77 ± 0.04 a | 0.78 ± 0.02 a | 0.76 ± 0.02 a | 0.78 ± 0.05 a | ||||||||||||||||||||||||||||||||
| O | 6 | Catechin, | Epicatechin | |||||||||||||||||||||||||||||||||||
| 6.35 | 470.1659 [M + H] | + | 492.1486 [M + Na] | + | 468.1540 [M − H] | − | 937.3156 [2M − H] | − | 469 | 292.1217, 424.1651 |
Unidentified | 463.0912 [M − H] | − | 464 | 301.0380 178.9999 151.0046 |
C | 21 | H | 20 | O | 11 | Isoquercitirin Hyperoside |
||||||||||||||||
| 7.48 | 435.0926 [M + H] | + | 457.0743 [M + Na] | + | 433.0801 [M − H] | − | 434 | −301.0381 | C | 20 | H | 18 | O | 11 | Reynoutrin Quercetin 3-O-α-D-arabinopyranoside Quercetin 3-O-xyloside |
|||||||||||||||||||||||
| 551.1037 [M + H] | + | 573.0854 [M + Na] | + | 549.0919 | 550 | (−)-Rubrichalcolactone | Color (CIElab*) | L* | 69.67 ± 0.88 a | 67.13 ± 1.08 a | 68.21 ± 1.20 a | 71.83 ± 0.82 a | 58.17 ± 1.03 b | 43.14 ± 1.10 c | ||||||||||||||||||||||||
| 6.43 | 355.1024 | a* | ||||||||||||||||||||||||||||||||||||
| 505.1018 [M − H] | − | 504 | (6-(5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxo-4H-chromen-8-yl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl acetate | |||||||||||||||||||||||||||||||||||
| 7.59 | 449.1081 [M + H] | + | 447.0963 [M − H] | − | 448 | −301.0378 | C | 21 | H | 20 | O | 11 | Quercetin 3-O-α-D-rhamnopyranoside | 10.66 ±1.28 b | 14.19 ± 1.81 a | 13.43 ± 1.89 a | 10.64 ± 0.78 c | 29.44 ± 1.04 b | 41.14 ± 0.76 a | |||||||||||||||||||
| 7.76 | 353.1361 [M + H] | + | 376.2120 [M + Na] | + | 351.1412 [M − H] | − | 375.1473 [M + Na−H] | − | 352 | 335.1254 | C | 22 | H | 40 | O | 3 | b* | 19.36 ± 0.63 a | 20.05 ± 1.38 a | 17.80 ± 1.31 b | 18.57 ± 0.48 a | 11.70 ± 0.59 b | 11.10 ± 0.28 b | |||||||||||||||
| C | 22.10 ± 1.43 a | 24.56 ± 2.28 a | 22.31 ± 2.30 a | 21.07 ± 2.47 c | 31.87 ± 0.98 b | 42.61 ± 0.77 a | ||||||||||||||||||||||||||||||||
| h° | 61.16 ± 0.56 a | 54.71 ± 1.03 b | 52.97 ± 1.17 b | 60.27 ± 2.30 a | 21.92 ± 1.49 b | 15.12 ± 0.33 c | ||||||||||||||||||||||||||||||||
Data correspond to the mean ± SE. Different letters point to significant differences between developmental stages in each harvest season (p ≤ 0.05). SSC, soluble solids content; TA, titratable acidity; FW, fresh weight; L/D, length and diameter ratio; DW, dry weight. Statistical analysis: One-Way ANOVA. Tukey (p < 0.05). nd*, not detected.
The pH range during the development of peumo fruits was 5.6–6.3. Titratable acidity (TA) could not be determined, as no drastic changes were detected in the titration curve. A significant soluble solids content (SSC) increase was observed only for the 2017 season from the Ca1 to Ca2 stages, with an SSC of 24–38° Brix in ripe peumo fruit (Table 1).
Concerning color changes during fruit development of the peumo (Table 1), the hue angle (h°) displayed a significant decrease during ripening in both seasons. In contrast, the L* and b* decreased significantly only in the second season. A higher increase of a* in the ripe stage was observed in the 2018 season. Nevertheless, the instrumental color obtained suggests the increase in red color during peumo ripening; the obtained data were not precise according to the visual color (Figure 1).
In the present study, the CO2 production of fruits decreases continually until the end of ripening during both seasons (Table 1). Indeed, ethylene production was not detected at any stage of peumo fruits (Table 1) in both seasons, suggesting a fruit’s non-climacteric behavior.
| cryptorigidifoliol A |
| 7.96 | ||||||||||||||
| 463.1238 | ||||||||||||||
| [M + H] | ||||||||||||||
| + | ||||||||||||||
| 461.1119 | ||||||||||||||
| [M − H] | ||||||||||||||
| − | ||||||||||||||
| 462 | ||||||||||||||
| C | 22 | H | 22 | O | 11 | Isorhamnetin-3-O-rhamnoside Luteolin 7-O-glucuronide |
||||||||
| 8.30 | 360.2155 [M + H] | + | 359.1525 | 358 | 313.1465, 327.1466, 341.1624 |
C | 20 | H | 24 | O | 6 | (+)-Lariciresinol 4-O-Methylcedrusin |
||
| 8.93 | 263.1641 [M + H] | + | 285.1458 [M + Na] | + | 262 | 165.0546 | C | 16 | H | 22 | O | 3 | 1′R*,3′S*,4′R*,5′S*,6S-6-[(4′-ethyl-9′-oxabicycle[3.3.1]non-6′-en-3′-yl)methyl]- 5,6- dihydro-2H-pyran-2-one |
|
| 9.21 | 376.2599 [M + H] | + | 398.2415 [M + Na] | + | 374.2470 [M − H] | − | 375 | 209.1284 275.1754 293.1861 302.1864 |
Unidentified | |||||
| 9.44 | 315.1620 | −118.0428 −163.0409 −271.1726 |
C | 16 | H | 12 | O | 7 | Sexangularetin | |||||
| 10.31 | 305.1747 [M + H] | + | 327.1568 [M + Na] | + | 304 | Unidentified | ||||||||
| 10.75 | 247.1694 [M + H] | + | 269.1513 [M + Na] | + | 246 | 173.1328 229.1592 |
C | 15 | H | 18 | O | 3 | Ethyl 5-hydroxy-7-phenyl-2,6-heptadienoate | |
| 11.26 | 249.1850 [M + H] | + | 271.1668 [M + Na] | + | 248 | 133.1016 231.1750 |
C | 16 | H | 24 | O | 2 | (4R,6S)-10-Phenyl-1-decene-4,6-diol | |
| Antioxidant capacity of peumo extract | ||||||||||||||
| ORAC (mmol/g DW | 0.637 ± 0.061 | |||||||||||||
| TPC (mgGAE/gDW) | 23.81 ± 3.06 | |||||||||||||
| TFC (mgQE/gDW) | 18.84 ± 3.33 | |||||||||||||
References
- Fuentes-Ramírez, A.; Pauchard, A.; Cavieres, L.A.; García, R.A. Survival and growth of Acacia dealbata vs. native trees across an invasion front in south-central Chile. For. Ecol. Manag. 2011, 261, 1003–1009.
- Benedetti, S. Información Tecnológica de Productos Forestales No Madereros del Bosque Nativo en Chile; Monografía de peumo Cryptocarya alba (Mol) Looser; CONAF: Santiago, Chile, 2012; ISBN 978-956-318-066-4. Available online: https://investigacion.conaf.cl/archivos/repositorio_documento/2018/11/004_2011-DOCUMENTOS_MONOGRAFIA_PEUMO.pdf (accessed on 28 October 2021).
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition. In Saponins in Food, Feedstuffs and Medicinal Plants; Oleszek, W., Marston, A., Eds.; Proceedings of the Phythochemical Society of Europe book Series; Springer: Dordrecht, The Netherlands, 2000; Volume 45.
- Fuentes-Barros, G.; Castro-Saavedra, S.; Liberona, L.; Acevedo-Fuentes, W.; Tirapegui, C.; Mattar, C.; Cassels, B.K. Variation of the alkaloid content of Peumus boldus (boldo). Fitoterapia 2018, 127, 179–185.
- Vogel, H.; Razmilic, I.; San Martín, J.; Doll, U.; y González, B. Plantas Medicinales Chilenas. Experiencia de Domesticación y Cultivo de Boldo, Matico, Bailahuén, Canelo, Peumo y Maqui, 2nd ed.; Editorial de la Universidad de Talca: Talca, Chile, 2008; 194p.
- Avello-Lorca, M.; López Canales, C.; GaticaValenzuela, C.; Bustos Concha, E.; Chait, A.B.; Pastene Navarrete, C.E.; Bittner Berner, C.M. Antimicrobial effects of extracts from Chilean plants of Lauraceae and Atherospermataceae families. Rev. Cub. Plant. Med. 2012, 17, 73–83.
- Schmeda-Hirschmann, G.; Astudillo, L.; Bastida, J.; Codina, C.; de Arias, A.R.; Ferreira, M.E.; Inchaustti, A.; Yaluff, G. Cryptofolione derivatives from Cryptocarya alba fruits. J. Pharm. Pharmacol. 2010, 53, 563–567.
- Domínguez, S.Y.; Martínez, E. Árboles de nuestros bosques. In Guía Didáctica; Ediciones Alfonso Martínez, S. L: Madrid, Spain, 1999.
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080.
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859.
- Ruiz, A.; Hermosí;n-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; Von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis microphylla) Fruits and Other Native Berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089.
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M.; Vinet, R. Patagonian Berries: Healthy Potential and the Path to Becoming Functional Foods. Foods 2019, 8, 289.
- Céspedes, C.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilenesis (Elaeocarpaceae). Maqui. Food Chem. 2008, 108, 820–829.
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.A.L.F.; Leighton, F. Juice and Phenolic Fractions of the Berry Aristotelia chilensis Inhibit LDL Oxidation in Vitro and Protect Human Endothelial Cells against Oxidative Stress. J. Agric. Food Chem. 2002, 50, 7542–7547.
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; de Mejia, E.G. Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976.
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving human health and healthy aging, and promoting quality life A review. Plant Foods Hum. Nutr. 2010, 65, 299–308.
More
