Advancements in high-throughput technology provide new opportunities for omics research to understand the pathological process of various complex human diseases. The integration of multi-omics technologies can systematically reveal the interactions among aging molecules from a multidimensional perspective.
- aging
- aging biomarkers
- antiaging targets
- multi-omics
- aging clock
1. Introduction
2. The Necessity of Distinguishing Chronological Age and Biological Age
3. Multi-Omics for Aging Clocks
3.1. Epigenetics Aging Clocks
3.2. Transcriptomics Aging Clocks
3.3. Proteomics Aging Clocks
3.4. Metabolomics Aging Clocks
3.5. Microbiomics Aging Clocks
4. Multi-Omics Approach for the Discovery of Aging Biomarkers
Aging is the main risk factor for chronic diseases that limits a healthy lifespan. Therefore, the mechanism of aging is a potential therapeutic target. Age correlation analyses involve large amounts of data obtained from various omics analyses, such as genomics (epigenomics), transcriptomics, proteomics, metabolomics, and microbiomics. We elaborated in detail in the Wu L. et al. [45] literature, 4.1 Aging Genomics, 4.1.1 Aging Epigenomics, 4.1.2 Aging Gene Expression, 4.1.3 Telomere-Based Biomarkers, 4.2 Aging Transcriptomics, 4.2.1Transcriptomics -Based Biomarkers, 4.2.2 MiRNAs, lncRNAs, and circRNAs-Based Biomarkers, 4.3 Aging Proteomics, 4.3.1 Proteomics-Based Biomarkers, 4.3.2 Senescence-Associated Secretory Phenotype-Based Biomarkers, 4.4 Aging Metabolomics, 4.5 Aging Microbiomics, 4.6 Early Biomarkers of AgingThe main advantages of this method include the analysis of all possible data pertaining to a single person or a large group of people, as well as the common and individual characteristics from a multi-dimensional perspective and the identification of aging markers and novel antiaging targets. Machine learning methods based on deep neural networks are the latest and most complex methods for identifying human aging biomarkers. They can utilize any type of omics data to predict age.
5. Integromicsand Systems Biology
To promote the multidimensional analysis of data, advanced omics technology is inseparable from advanced omics analytical tools. At present, large-scale, high-quality, and high-throughput data from various omics methods can be efficiently and independently analyzed. However, separate data analysis and interpretation ignore the correlation and biological interference between different omics levels. Therefore, the integration of single-omics methods is essential for an in-depth understanding of the aging process and its mechanism.
Integromics, the comprehensive analysis of different omics data, and systems biology have provided several breakthroughs in the study of aging and antiaging interventions. Together, they have emerged as a more complex statistical method and combine the experimental data obtained in multiple omics methods with computational models to provide a holistic view of the aging landscape [46]. Considering the complexity and heterogeneity of aging, integromics and systems biology not only provide static maps of molecules but are also used to characterize the mutual changes of molecules over time. This helps determine the optimal time point for aging biomarker measurements and specific antiaging drug treatments. Each omics-level biomarker candidate based on integromics and systems biology has biological relevance. Significant biomarker candidates can be preferentially used as biomarkers of aging in medicine and as new antiaging targets.
6. Conclusions and Prospects
Rapid advances in science and technology have accelerated the arrival of the “omics era”, thereby enabling researchers to collect and integrate data at different molecular levels. The identification of biomarkers of aging and new targets for antiaging interventions is crucial in aging biology and geriatrics. The multi-level information obtained through multi-omics technology contributes to the increased understanding of the mechanisms of aging and provides new opportunities for the diagnosis and treatment of aging and aging-related diseases.
We have summarized the various omics techniques used to characterize aging biomarkers. Each screened biomarker is a promising candidate and can be integrated into an “aging biomarker library” that can serve as a diagnostic and prognostic tool. Here, we mainly categorized them based on the existing biomarkers of aging. We summarized the recent omics methods used to discover biomarkers in genomics, transcriptomics, proteomics, metabolomics, and metagenomics (Figure 1).
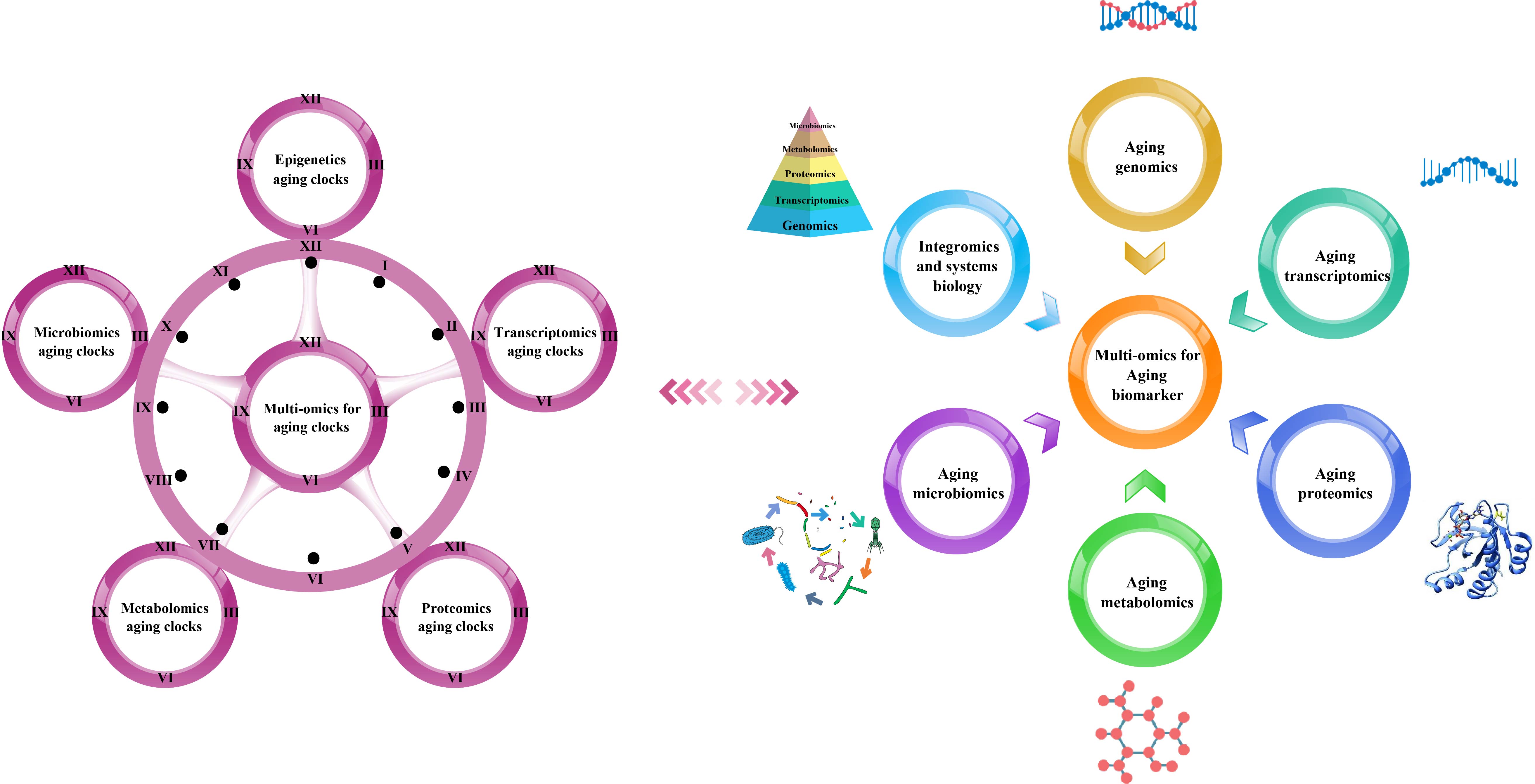
Figure 1. Multi-omics-based technologies for characterizing aging clocks and biomarkers. Aging is a comprehensive process affected by multiple factors that is associated with changes at the molecular, cellular, tissue, and organism levels, thus requiring objective analytical research tools. The integrated multi-omics approach is essential to achieve a comprehensive understanding of the biological mechanisms of aging.
In the context of personalized and precision medicine, multi-omics methods have attracted widespread attention, because they can provide an in-depth understanding of the molecular patterns and cover a wide range of characteristics, such as participating in the metabolic, genetic, and signal transduction pathways of complex aging [47]. Therefore, we suggest that a combination of multiple biomarkers for a comprehensive diagnosis and systematic analysis can objectively characterize the aging process (Figure 2).
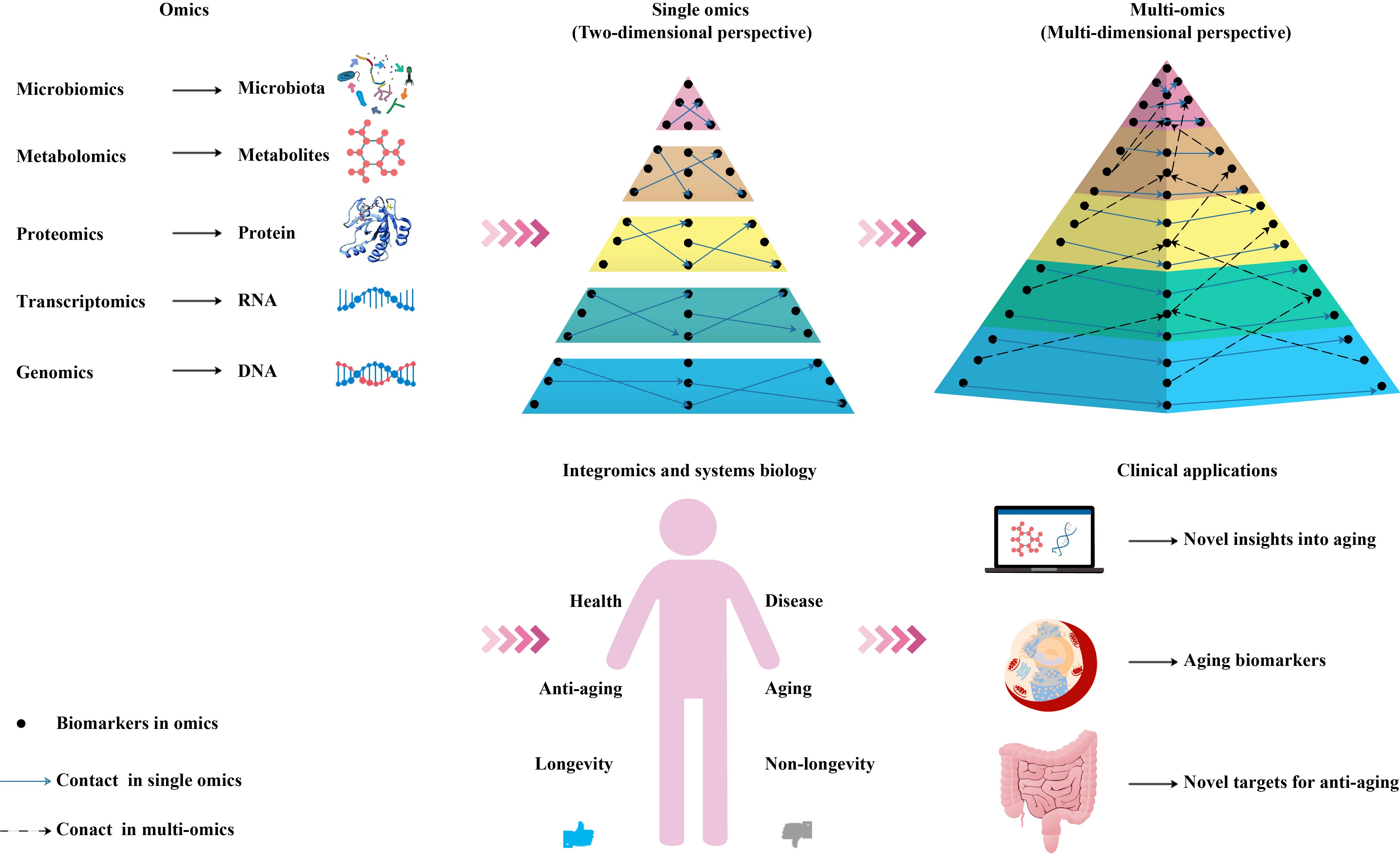
Figure 2. Schematic diagram of an integrated multi-omics approach to the research and application of aging biomarkers. Genomics, transcriptomics, proteomics, metabolomics, and microbiomics enable the high-throughput quantitative profiling of molecules in biological systems to reveal aging-related changes. Combining single-omics data with integromics and systems biology contributes to an increased understanding of the mechanisms of aging and paves the way for the development and utilization of aging biomarkers and novel antiaging targets.
Advances in computer science, including meta-analysis and artificial intelligence, are expected to remarkably increase the speed and efficiency of aging biomarker research [48]. However, before their application in the clinical setting, candidate biomarkers should be verified. This verification process must include larger sample populations. Despite the large gap between the identification of useful biomarkers and their application in clinical practice, the integrated analysis of multi-omics data is a promising tool to identify new candidate biomarkers that could be developed and used to identify pharmaceutical targets and improve human health during aging, thereby advancing our understanding of the pathophysiology of the complex and dynamic process of aging.
References
- World Population Prospects 2019: Highlights . Department of Economic and Social Affairs, Population Division. Retrieved 2022-1-7
- Carmona, J.J.; Michan, S.; Biology of healthy aging and longevity. Rev. Investig. Clin.-Clin. Transl. Investig. 2016, 68, 7-16.
- Tuttle, C.S.L.; Waaijer, M.E.C.; Slee-Valentijn, M.S.; Stijnen, T.; Westendorp, R.; Maier, A.B.; Cellular senescence and chronological age in various human tissues: A systematic review and meta-analysis. Aging Cell 2020, 19, 1-11, https://doi.org/10.1111/acel.13083.
- Denic, A.; Glassock, R.J.; Rule, A.D.; Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 2016, 23, 19-28, https://doi.org/10.1053/j.ackd.2015.08.004.
- Kim, I.H.; Kisseleva, T.; Brenner, D.A.; Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184-191, https://doi.org/10.1097/mog.0000000000000176.
- Bernardes de Jesus, B.; Blasco, M.A.; Potential of telomerase activation in extending health span and longevity. Curr. Opin. Cell Biol. 2012, 24, 739-743, https://doi.org/10.1016/j.ceb.2012.09.004.
- Gross, A.L.; Carlson, M.C.; Chu, N.M.; McAdams-DeMarco, M.A.; Mungas, D.; Simonsick, E.M.; Derivation of a measure of physiological multisystem dysregulation: Results from WHAS and health ABC. Mech. Ageing Dev. 2020, 188, 111258, https://doi.org/10.1016/j.mad.2020.111258.
- Belloni, G.; Cesari, M.; Frailty and intrinsic capacity: Two distinct but related constructs. Front. Med. 2019, 6, 133, https://doi.org/10.3389/fmed.2019.00133.
- Zhavoronkov, A.; Buzdin, A.A.; Garazha, A.V.; Borisov, N.M.; Moskalev, A.A.; Signaling pathway cloud regulation for in silico screening and ranking of the potential geroprotective drugs. Front. Genet. 2014, 5, 49, https://doi.org/10.3389/fgene.2014.00049.
- Gott, A.; Andrews, C.; Hormigos, M.L.; Spencer, K.; Bateson, M.; Nettle, D.; Chronological age, biological age, and individual variation in the stress response in the European starling: A follow-up study. PeerJ 2018, 6, e5842, https://doi.org/10.7717/peerj.5842.
- Brown, P.J.; Wall, M.M.; Chen, C.; Levine, M.E.; Yaffe, K.; Roose, S.P.; Rutherford B.R.; Rutherford B.R. Biological age, not chronological age, is associated with late-life depression. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2018, 73, 1370–1376, https://doi.org/10.1093/gerona/glx162.
- Kim, S.J.; Kim, B.J.; Kang, H.; Measurement of biological age may help to assess the risk of colorectal adenoma in screening colonoscopy. World J. Gastroenterol. 2017, 23, 6877–6883, https://doi.org/10.3748/wjg.v23.i37.6877.
- Cho, I.H.; Park, K.S.; Lim, C.J.; An empirical comparative study on biological age estimation algorithms with an application of Work Ability Index (WAI). Mech. Ageing Dev. 2010, 131, 69–78, https://doi.org/10.1016/j.mad.2009.12.001.
- Klemera, P.; Doubal, S.; A new approach to the concept and computation of biological age. Mech. Ageing Dev. 2006, 127, 240–248, https://doi.org/10.1016/j.mad.2005.10.004.
- Bai, X.J.; Han, L.L.; Liu, Q.; Shan, H.Y.; Lin, H.L.; Sun, X.F.; Chen, X.M.; Evaluation of biological aging process—A population-based study of healthy people in china. Gerontology 2010, 56, 129–140, https://doi.org/10.1159/000262449.
- Zhang, W.G.; Bai, X.J.; Sun, X.F.; Cai, G.Y.; Bai, X.Y.; Zhu, S.Y.; Zhang, M.; Chen, X. M.; Construction of an integral formula of biological age for a healthy Chinese population using principle component analysis. J. Nutr. Health Aging 2014, 18, 137–142, https://doi.org/10.1007/s12603-013-0345-8.
- Belsky, D.W.; Caspi, A.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; Danese, A.; Harrington, H.; Israel, S.; Levine, M.E.; Schaefer, J.D.; et al. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110, https://doi.org/10.1073/pnas.1506264112.
- Mitnitski, A.; Howlett, S.E.; Rockwood, K.; Heterogeneity of human aging and its assessment. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2017, 72, 877–884, https://doi.org/10.1093/gerona/glw089.
- Horvath, S.; Raj, K.; DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384, https://doi.org/10.1038/s41576-018-0004-3.
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al.et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging-Us 2018, 10, 573–591, https://doi.org/10.18632/aging.101414.
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al.et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cel 2013, 49, 359–367, https://doi.org/10.1016/j.molcel.2012.10.016.
- Liu, Z.; Leung, D.; Levine, M.; Comparative analysis of epigenetic aging clocks from CpG characteristics to functional associations. bioRxiv 2019, 51, 512483, https://doi.org/10.1101/512483.
- Zhang, Y.; Wilson, R.; Heiss, J.; Breitling, L.P.; Saum, K.U.; Schoettker, B.; Holleczek, B.; Waldenberger, M.; Peters, A.; Brenner, H.; et al. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun. 2017, 8, 1-11, https://doi.org/10.1038/ncomms14617.
- Horvath, S.; Oshima, J.; Martin, G.M.; Lu, A.T.; Quach, A.; Cohen, H.; Felton, S.; Matsuyama, M.; Lowe, D.; Kabacik, S.; et al.et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging-Us 2018, 10, 1758–1775, https://doi.org/10.18632/aging.101508.
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sanchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E.; Epigenetic Predictor of Age. PLoS ONE 2011, 6, e14821, https://doi.org/10.1371/journal.pone.0014821.
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.F.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al.et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging-Us 2019, 11, 303–327, https://doi.org/10.18632/aging.101684.
- Wang, M.; Lemos, B.; Ribosomal DNA harbors an evolutionarily conserved clock of biological aging. Genome Res. 2019, 29, 325–333, https://doi.org/10.1101/gr.241745.118.
- Peters, M.J.; Joehanes, R.; Pilling, L.C.; Schurmann, C.; Conneely, K.N.; Powell, J.; Reinmaa, E.; Sutphin, G.L.; Zhernakova, A.; Schramm, K.; et al.et al. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 2015, 6, 1-14, https://doi.org/10.1038/ncomms9570.
- Fleischer, J.G.; Schulte, R.; Tsai, H.H.; Tyagi, S.; Ibarra, A.; Shokhirev, M.N.; Huang, L.; Hetzer, M.; Navlakha, S.; Predicting age from the transcriptome of human dermal fibroblasts. Genome Biol. 2018, 19, 1-18, https://doi.org/10.1186/s13059-018-1599-6.
- Putin, E.; Mamoshina, P.; Aliper, A.; Korzinkin, M.; Moskalev, A.; Kolosov, A.; Ostrovskiy, A.; Cantor, C.; Vijg, J.; Zhavoronkov, A.; et al. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging-Us 2016, 8, 1021–1033, https://doi.org/10.18632/aging.100968.
- Horvath, S.; DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, 1-20, https://doi.org/10.1186/gb-2013-14-10-r115.
- Mamoshina, P.; Kochetov, K.; Putin, E.; Aliper, A.; Zhavoronkov, A.; Testing for batch effect through age predictors. bioRxiv 2019, 1, 531863, https://doi.org/10.1101/531863.
- Bathke, J.; Konzer, A.; Remes, B.; McIntosh, M.; Klug, G.; Comparative analyses of the variation of the transcriptome and proteome of Rhodobacter sphaeroides throughout growth. BMC Genom. 2019, 20, 1-13, https://doi.org/10.1186/s12864-019-5749-3.
- Haider, S.; Pal, R.; Integrated analysis of transcriptomic and proteomic data. Curr. Genom. 2013, 14, 91–110, https://doi.org/10.2174/1389202911314020003.
- Tin, A.; Yu, B.; Ma, J.; Masushita, K.; Daya, N.; Hoogeveen, R.C.; Ballantyne, C.M.; Couper, D.; Rebholz, C.M.; Grams, M.E.; et al.et al. Reproducibility and variability of protein analytes measured using a multiplexed modified aptamer assay. J. Appl. Lab. Med. 2019, 4, 30–39, https://doi.org/10.1373/jalm.2018.027086.
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.B.; Cheung, F.; Fantoni, G.; et al.et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799, https://doi.org/10.1111/acel.12799.
- Johnson, A.A.; Shokhirev, M.N.; Wyss-Coray, T.; Lehallier, B.; Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Res. Rev. 2020, 60, 101070, https://doi.org/10.1016/j.arr.2020.101070.
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Losada, P.M.; Berdnik, D.; Keller, A.; Verghese, J.; et al.et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850, https://doi.org/10.1038/s41591-019-0673-2.
- Hertel, J.; Friedrich, N.; Wittfeld, K.; Pietzner, M.; Budde, K.; Van der Auwera, S.; Lohmann, T.; Teumer, A.; Voelzke, H.; Nauck, M.; et al.et al. Measuring biological age via metabonomics: The metabolic age score. J. Proteome Res. 2016, 15, 400–410, https://doi.org/10.1021/acs.jproteome.5b00561.
- van den Akker, E.B.; Trompet, S.; Wolf, J.J.H.B.; Beekman, M.; Suchiman, H.E.D.; Deelen, J.; Predicting biological age based on the BBMRI-NL 1H-NMR metabolomics repository. bioRxiv 2019, 1, 632919, https://doi.org/10.1101/632919.
- Choi, J.; Hur, T.Y.; Hong, Y.; Influence of altered gut microbiota composition on aging and aging-related diseases. J. Lifestyle Med. 2018, 8, 1-7, https://doi.org/10.15280/jlm.2018.8.1.1.
- Galkin, F.; Mamoshina, P.; Aliper, A.; Putin, E.; Moskalev, V.; Gladyshev, V.N.; Zhavoronkov, A.; Human gut microbiome aging clock based on taxonomic profiling and deep learning. Iscience 2020, 23, 101199, https://doi.org/10.1016/j.isci.2020.101199.
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O'Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O'Sullivan, O.; et al.et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184, https://doi.org/10.1038/nature11319.
- Woodmansey, E.J.; McMurdo, M.E.T.; Macfarlane, G.T.; Macfarlane, S.; Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl. Environ. Microbiol. 2004, 70, 6113–6122, https://doi.org/10.1128/aem.70.10.6113-6122.2004.
- Wu, L.; Xie, X.Q.; Liang, T.T.; Ma, J.; Yang, L.S.; Yang, J.; Li, L.Y.; Xi, Y.; Li, H.X.; Zhang, J.M.; et al. Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets. Biomolecules 2022, 12, 39, https://doi.org/10.3390/biom12010039.
- Cisek, K.; Krochmal, M.; Klein, J.; Mischak, H.; The application of multi-omics and systems biology to identify therapeutic targets in chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 2003–2011, https://doi.org/10.1093/ndt/gfv364.
- Solovev, I.; Shaposhnikov, M.; Moskalev, A.; Multi-omics approaches to human biological age estimation. Mech. Ageing Dev. 2020, 185, 111192, https://doi.org/10.1016/j.mad.2019.111192.
- Balasubramanian, P.; Howell, P.R.; Anderson, R.M.; Aging and caloric restriction research: A biological perspective with translational potential. EBioMedicine 2017, 21, 37–44, https://doi.org/10.1016/j.ebiom.2017.06.015.
