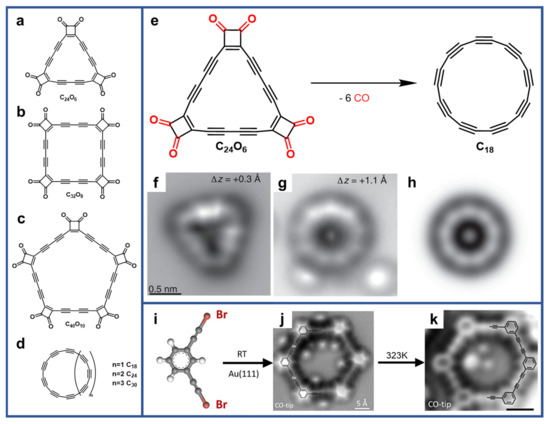
A plethora of novel two-dimensional carbon nanostructures are flourishing, such as nanoporous graphene, nonbenzenoid carbon allotropes, etc., especially the nanostructures involving
sp-hybridized carbons. The C–C triple bond is of great advantage for avoidance of fluctuation arising from
cis-
trans isomerization which is different from olefinic bond
[7][8][58,59]. What is more, the graphyne comprising of
sp- and
sp2-carbon atoms has been predicted to have a crystalline state, which was also predicted to be one of the most stable carbon phases containing acetylenic groups as a major structural component. Additionally, graphyne was calculated to be a semiconductor with a bandgap of 1.2 eV
[9][60], which demonstrated their possible applications in electronic devices. However, the atomic precise fabrication of graphyne is limited by its high chemical reactivity. Zhang et al. developed a method to grow extended 2D graphdiyne-like networks using terminal alkynes on Ag(111) via homocoupling reaction, as demonstrated in
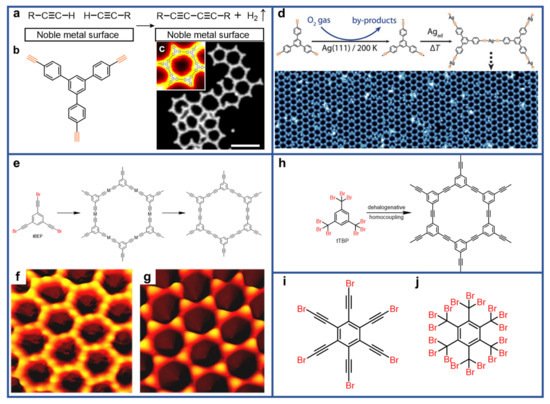
Figure 2a–c
[10][61]. The STM image in
Figure 2b revealed a closer inspection of irregular, open-porous networks. The inset showed a magnified image of the associated honeycomb unit, and a corresponding model. The formation of covalent bonds was substantiated by complementary XPS measurements. The absence of the low energy shoulder peak at 283.7 eV (a typical binding energy for methylacetylide) revealed a resulting covalent structure, which is contradictory to that proposed by the organometallic binding mechanism when compared with the simulated XPS spectrum of the organometallic dimer. Other impressive efforts have also been devoted to synthesizing graphyne-like nanostructures. Similarly, Zhang et al. have synthesized highly regular single-layer alkynyl-silver organometallic networks at the micrometer scale via gas-mediated surface reaction (
Figure 2d)
[11][62]. Different from the previous strategy through thermal induction combined with catalyzation via substrate, terminal alkyne radicals were obtained via oxygen gas mediated deprotonation on Ag(111), and the activation procedure was confirmed by XPS spectroscopy. The peak at 283.6 eV evidenced the strong interaction between the alkynyl groups and the substrates. There was an absence of the O 1s signature in the XPS spectrum after dosing O
2, ruling out adsorbed oxygen species and other intermediates containing oxygen. The results indicated that both gas species and substrate play crucial roles in the particular activation process. This work provides a versatile fabrication procedure featuring high chemoselectivity without poisoning the surface. Aside from dehydrogenation, debromination of
sp- and
sp3-carbon was explored, which greatly avoids the byproducts generated at elevated temperatures. As demonstrated in
Figure 2e–g, Xu group applied the on-surface synthesis protocol by introducing dehalogenative homocouplings of alkynyl bromides on Au(111), which results in the formation of an organometallic intermediate and subsequent release of gold atoms at elevated temperature
[12][51]. Furthermore, they synthesized C≡C triple-bonded structural motif by dehalogenative homocouplings of tribromomethyl-substituted arenes, as illustrated in
Figure 2h
[13][52]. Notably, the organometallic intermediates were not formed in this process. This work provides a new protocol to convert
sp3-carbon atoms to
sp-hybridized ones through pre-designated molecular precursor. Moreover,
Figure 2i,j demonstrate two potential precursors that could be considered to synthesize graphdiyne and graphyne, respectively.
Figure 2. (
a) Scheme of surface-assisted Glaser homocoupling reaction. (
b) Chemical structure of 1,3,5-tris-(4-ethynyl phenyl)benzene (TEPB). (
c) An open reticular structure from the merged ethynyl moieties prevails. The inset shows a single honeycomb nanopore superimposed with a calculated model. Reprinted with permission from Ref.
[10][61]. Copyright 2012, Macmillan Publisher Limited. (
d) Reaction scheme and STM image of single-layer alkynyl−silver networks at the micrometer scale via gas-mediated surface reaction. Reprinted with permission from Ref.
[11][62]. Copyright 2019, American Chemical Society. (
e) Schematic illustration of dehalogenative homocoupling of 1,3,5-tris(2-bromoethynyl)benzene (tBEP). (
f,
g) STM images of the (
f) C–Au–C organometallic network and the (
g) C–C coupled network. Reprinted with permission from Ref.
[12][51]. Copyright 2016, American Chemical Society. (
h) Scheme of dehalogenative homocoupling of 1,3,5-tris(tribromomethyl)benzene (tTBP) molecule. Reprinted with permission from Ref.
[13][52]. Copyright 2018, Wiley-VCH Verlag GmbH &Co. KGaA, Weinheim. (
i,
j) Two potential precursors to synthesize graphdiyne and graphyne structures.
 Encyclopedia
Encyclopedia
