Animals adopt several strategies to regulate their body temperature by promoting heat loss or gain in hot and cold environments, respectively. This mechanism of heat loss or production is performed in thermal windows. A thermal window is a structure where many blood capillaries facilitate thermal exchange in this region. The presence of feathers, hair, or glabrous (hairless) skin and their structural characteristics greatly influence each species’ capacity to maintain thermal comfort. This factor needs to be considered when implementing new monitoring or measuring techniques such as infrared thermography since interpretations may vary due to the presence or absence of these structures. It is essential to recognize the effects of glabrous skin, hair, and feathers on thermoregulation to identify species-specific thermal windows that allow accurate evaluations of the thermal state of domestic animals.
- birds
- behavior
- thermoregulation
- skin
- glabrous skin
- hair
- feathers
- welfare
1. Introduction
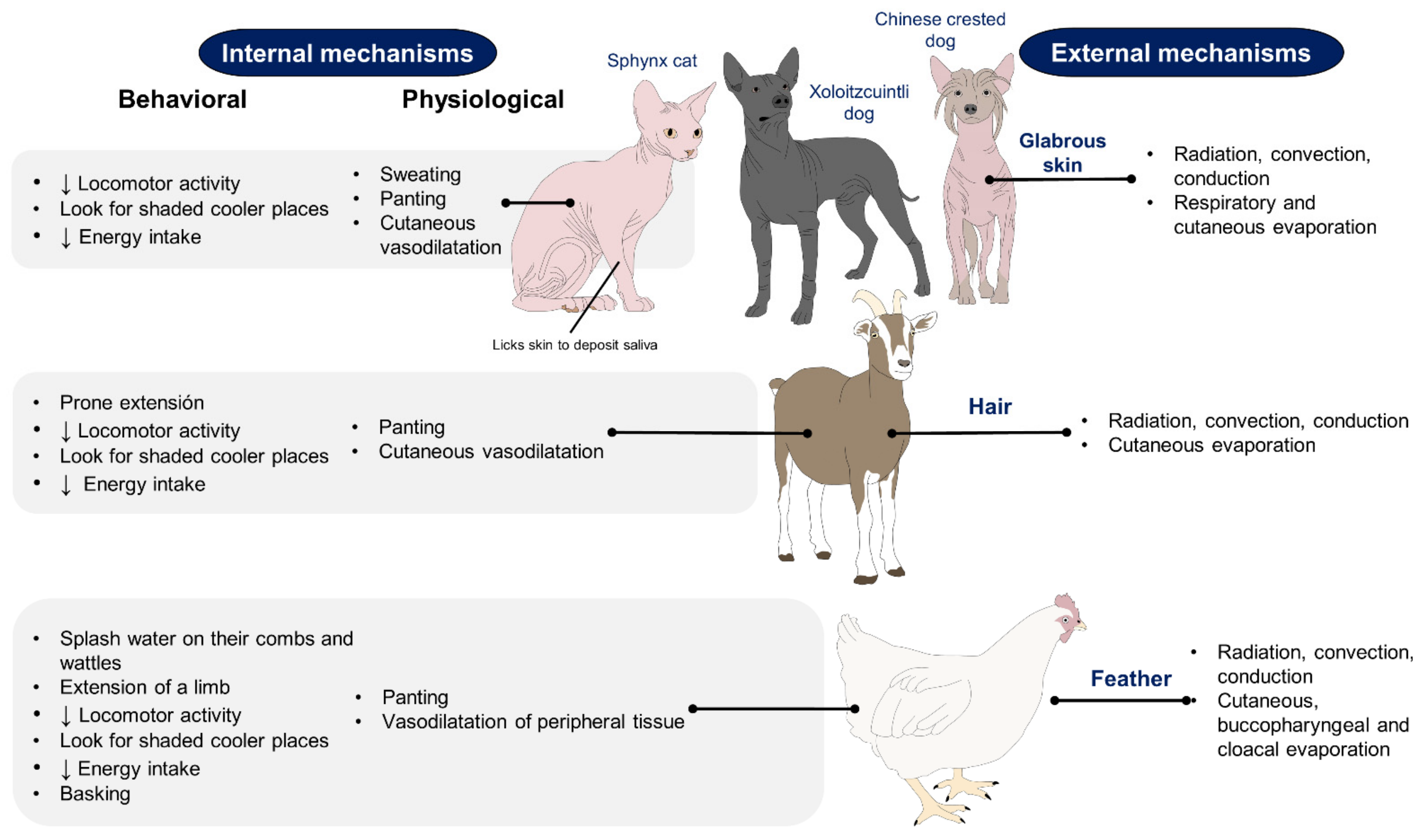
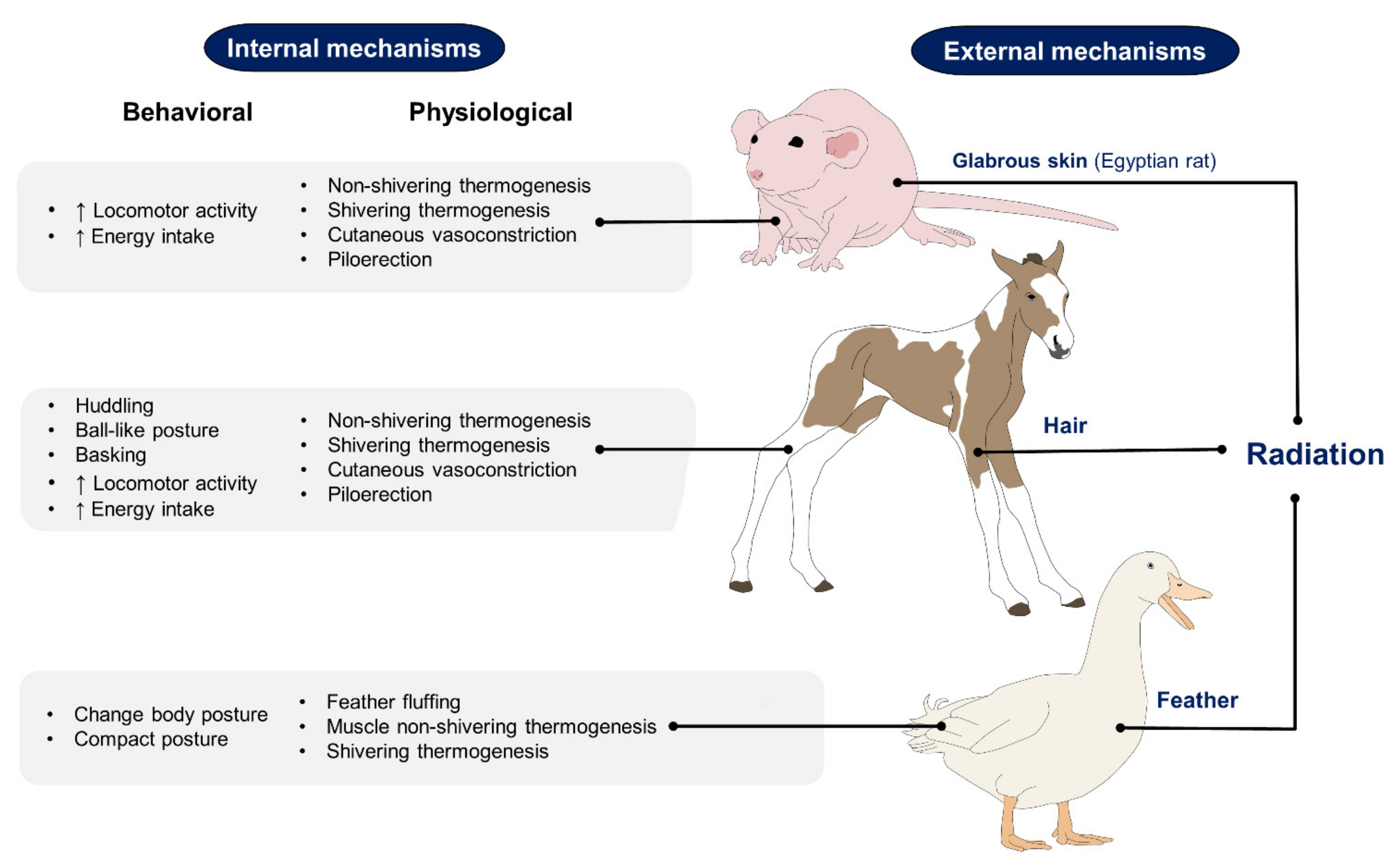
2. Effect of Hair, Feathers, and Glabrous Skin Based on Recent Scientific Findings on the Use of IRT
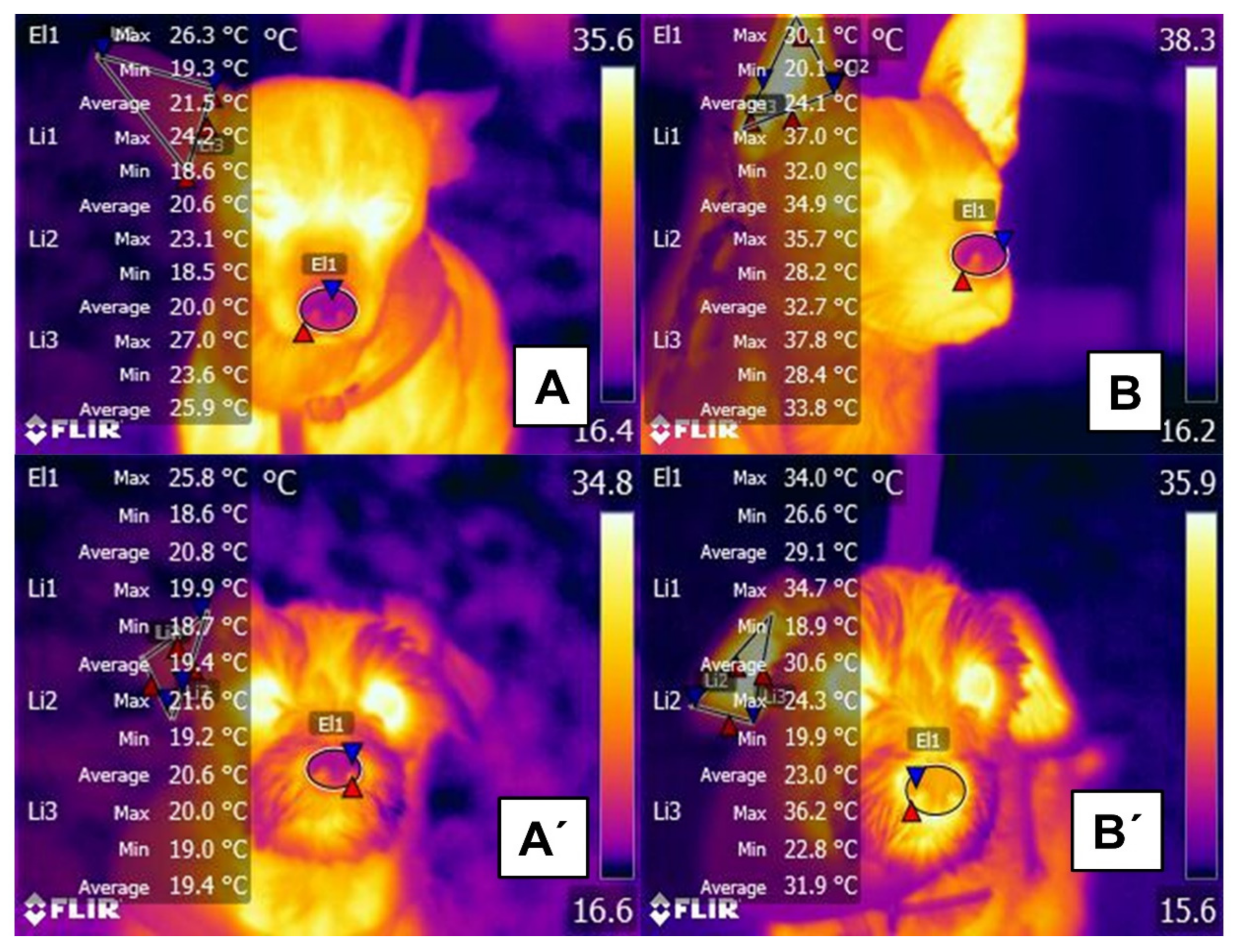
2.1. Hair
2.2. Feathers
Studies of animals with feathers using invasive techniques have correlated circulating cortisol levels with the ocular region’s temperatures under caloric stress conditions (Figure 74) [31]. It should be noted that the ocular region is widely used in domestic animals to determine the increase in body temperature in response to the activation of the sympathetic nervous system under stressful conditions such as stress hyperthermia [120][37]. This effect is observed as an increase in the superficial temperature of the orbital region [121,122][38][39].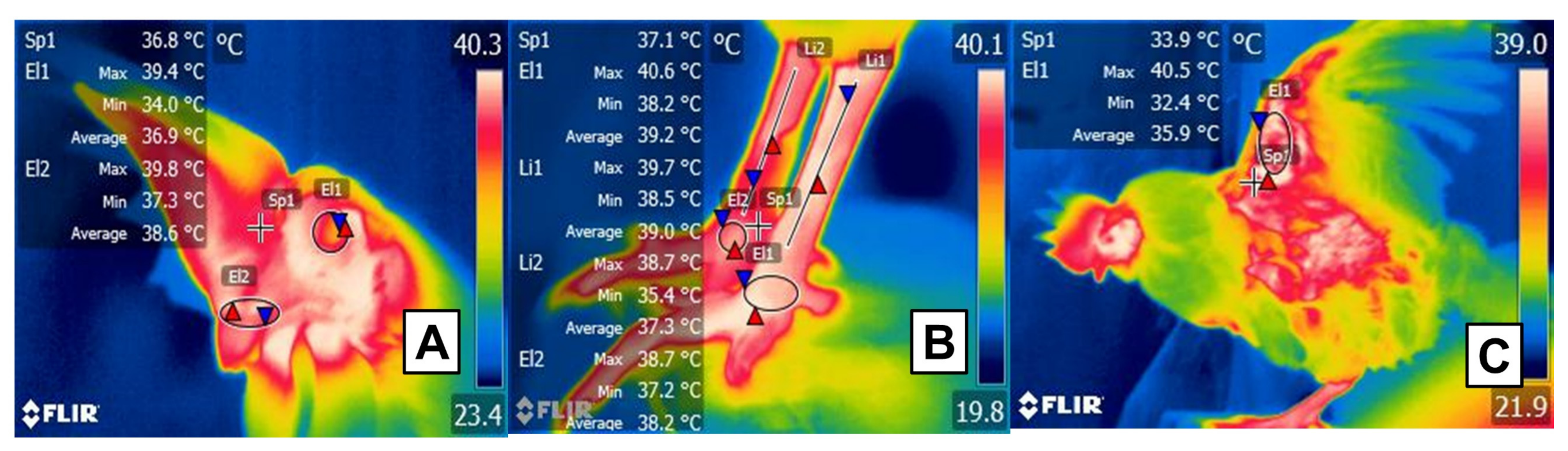
2.3. Glabrous Skin
3. Conclusions
References
- Fiebig, K.; Jourdan, T.; Kock, M.H.; Merle, R.; Thöne-Reineke, C. Evaluation of infrared thermography for temperature measurement in adult male NMRI nude mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 715–724.
- Mota-Rojas, D.; Orihuela, A.; Strappini-Asteggiano, A.; Nelly Cajiao-Pachón, M.; Agüera-Buendía, E.; Mora-Medina, P.; Ghezzi, M.; Alonso-Spilsbury, M. Teaching animal welfare in veterinary schools in Latin America. Int. J. Vet. Sci. Med. 2018, 6, 131–140.
- Szekely, M. Thermoregulation energy balance regulatory peptides recent developments. Front. Biosci. 2010, 2, 116.
- Bouchama, A.; Knochel, J.P. Heat stroke. N. Engl. J. Med. 2002, 346, 1978–1988.
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19.
- Kingma, B. The thermoneutral zone: Implications for metabolic studies. Front. Biosci. 2012, 4, 1975.
- Kingma, B.R.; Frijns, A.J.; Schellen, L.; van Marken Lichtenbelt, W.D. Beyond the classic thermoneutral zone. Temperature 2014, 1, 142–149.
- Schepers, R.J.; Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 2010, 34, 177–184.
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715.
- Señarís, R.; Ordás, P.; Reimúndez, A.; Viana, F. Mammalian cold TRP channels: Impact on thermoregulation and energy homeostasis. Pflügers Arch. Eur. J. Physiol. 2018, 470, 761–777.
- Kregel, K.C.; Wall, P.T.; Gisolfi, C.V. Peripheral vascular responses to hyperthermia in the rat. J. Appl. Physiol. 1988, 64, 2582–2588.
- Romanovsky, A.A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R37–R46.
- Cheshire, W.P. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104.
- Epstein, Y.; Moran, D.S. Thermal comfort and the heat stress indices. Ind. Health 2006, 44, 388–398.
- Mortola, J.P. Social interaction and the thermogenic response of chicken hatchlings. Physiol. Behav. 2021, 232, 113317.
- Robertshaw, D. Mechanisms for the control of respiratory evaporative heat loss in panting animals. J. Appl. Physiol. 2006, 101, 664–668.
- Ratnakaran, A.P.; Sejian, V.; Jose, V.S.; Vaswani, S.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Varma, G.; Bhatta, R. Behavioral responses to livestock adaptation to heat stress challenges. Asian J. Anim. Sci. 2016, 11, 1–13.
- Affolter, V.K.; Moore, P.F. Histologic features of normal canine and feline skin. Clin. Dermatol. 1994, 12, 491–497.
- Mecklenburg, L. An overview on congenital alopecia in domestic animals. Vet. Dermatol. 2006, 17, 393–410.
- Parker, H.G.; Harris, A.; Dreger, D.L.; Davis, B.W.; Ostrander, E.A. The bald and the beautiful: Hairlessness in domestic dog breeds. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20150488.
- Mauldin, E.A.; Peters-Kennedy, J. Integumentary system. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Grant, M.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 509–736.
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733.
- Precht, H.; Christophersen, J.; Hensel, H.; Larcher, W. Heat exchange with the environment. In Temperature and Life, 2nd ed.; Precht, H., Christophersen, J., Hensel, H., Larcher, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1973; pp. 545–564.
- Hillman, R.; Gilbert, R. Reproductive diseases. In Rebhun’s Diseases of Dairy Cattle; Divers, T.J., Peek, S.F., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2008; pp. 395–446.
- Gordon, C.J.; Becker, P.; Ali, J.S. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol. Behav. 1998, 65, 255–262.
- Yáñez-Pizaña, A.; de la Cruz-Cruz, L.A.; Tarazona-Morales, A.; Roldan-Santiago, P.; Ballesteros-Rodea, G.; Pineda-Reyes, R.; Orozco-Gregorio, H. Physiological and behavioral changes of water buffalo in hot and cold systems: Review. J. Buffalo Sci. 2020, 9, 110–120.
- Lalonde, S.; Beaulac, K.; Crowe, T.G.; Schwean-Lardner, K. The effects of simulated transportation conditions on the core body and extremity temperature, blood physiology, and behavior of white-strain layer pullets. Poult. Sci. 2021, 100, 697–706.
- Nowack, J.; Giroud, S.; Arnold, W.; Ruf, T. Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Front. Physiol. 2017, 8, 889.
- Tattersall, G.J.; Andrade, D.V.; Abe, A.S. Heat exchange from the toucan bill reveals a controllable vascular thermal radiator. Science 2009, 325, 468–470.
- Arfuso, F.; Fazio, F.; Rizzo, M.; Marafioti, S.; Zanghì, E.; Piccione, G. Factors affecting the hematological parameters in different goat breeds from Italy. Ann. Anim. Sci. 2016, 16, 743–757.
- Jerem, P.; Jenni-Eiermann, S.; Herborn, K.; McKeegan, D.; McCafferty, D.J.; Nager, R.G. Eye region surface temperature reflects both energy reserves and circulating glucocorticoids in a wild bird. Sci. Rep. 2018, 8, 1907.
- Mota-Rojas, D.; Pereira, M.F.A.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Ávalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical applications and factors involved in validating thermal windows in large rumiants to assess health and productivity. Animals 2021, 11, 2247.
- Allen, J.; Howell, K. Microvascular imaging: Techniques and opportunities for clinical physiological measurements. Physiol. Meas. 2014, 35, R91–R141.
- Rizzo, M.; Arfuso, F.; Alberghina, D.; Giudice, E.; Gianesella, M.; Piccione, G. Monitoring changes in body surface temperature associated with treadmill exercise in dogs by use of infrared methodology. J. Therm. Biol. 2017, 69, 64–68.
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5.
- Kwon, C.J.; Brundage, C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019, 82, 18–22.
- Mota-Rojas, D.; Miranda-Cortés, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato-Funes, L.; Hernández-Ávalos, I. Neurobiología y modulación de la hipertermia inducida por estrés agudo y fiebre en los animales. Abanico Vet. 2021, 11, 1–17.
- Church, J.; Cook, N.; Schaefer, A. Recent Applications of Infrared Thermography for Animal Welfare and Veterinary Research: Everything from Chicks to Elephants. In Proceedings of the Conference InfraMation, Las Vegas, NV, USA, 9 December 2009.
- Cook, N.J.; Schaefer, A.L.; Korver, D.R.; Haley, D.B.; Feddes, J.J.R.; Church, J.S. Minimally-invasive assessments of the behavioural and physiological effects of enriched colony cages on laying hens. Open Agric. J. 2011, 5, 10–18.
- Herborn, K.A.; Jerem, P.; Nager, R.G.; McKeegan, D.E.F.; McCafferty, D.J. Surface temperature elevated by chronic and intermittent stress. Physiol. Behav. 2018, 191, 47–55.
- Jerem, P.; Herborn, K.; McCafferty, D.; McKeegan, D.; Nager, R. Thermal imaging to study stress non-invasively in unrestrained birds. J. Vis. Exp. 2015, 2015, 53184.
- Powers, D.R.; Langland, K.M.; Wethington, S.M.; Powers, S.D.; Graham, C.H.; Tobalske, B.W. Hovering in the heat: Effects of environmental temperature on heat regulation in foraging hummingbirds. R. Soc. Open Sci. 2017, 4, 171056.
- Mitchell, W.F.; Clarke, R.H. Using infrared thermography to detect night-roosting birds. J. F. Ornithol. 2019, 90, 39–51.
- Loyau, T.; Zerjal, T.; Rodenburg, T.B.; Fablet, J.; Tixier-Boichard, M.; Pinard-van der Laan, M.H.; Mignon-Grasteau, S. Heritability of body surface temperature in hens estimated by infrared thermography at normal or hot temperatures and genetic correlations with egg and feather quality. Animal 2016, 10, 1594–1601.
- Barnett, J.L.; Hemsworth, P.H.; Cronin, G.M.; Jongman, E.C.; Hutson, G.D. A review of the welfare issues for sows and piglets in relation to housing. Aust. J. Agric. Res. 2001, 52, 1–28.
- Kammersgaard, T.S.; Pedersen, L.J.; Jorgensen, E. Hypothermia in neonatal piglets: Interactions and causes of individual differences. J. Anim. Sci. 2011, 7, 2073–2085.
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45.
- Schmitt, O.; Reigner, S.; Bailly, J.; Ravon, L.; Billon, Y.; Gress, L.; Bluy, L.; Canario, L.; Gilbert, H.; Bonnet, A.; et al. Thermoregulation at birth differs between piglets from two genetic lines divergent for residual feed intake. Animal 2021, 15, 100069.
