Natural emulsion stabilizers are polymers of amino acid, nucleic acid, carbohydrate, etc., which are derived from microorganisms, bacteria, and other organic materials. Plant and animal proteins are basic sources of natural emulsion stabilizers. Pea protein-maltodextrin and lentil protein feature entrapment capacity up to 88%, (1–10% concentrated), zein proteins feature 74–89% entrapment efficiency, soy proteins in various concentrations increase dissolution, retention, and stability to the emulsion and whey proteins, egg proteins, and proteins from all other animals are applicable in membrane formation and encapsulation to stabilize emulsion/nanoemulsion. In pharmaceutical industries, phospholipids, phosphatidyl choline (PC), phosphatidyl ethanol-amine (PE), and phosphatidyl glycerol (PG)-based stabilizers are very effective as emulsion stabilizers. Lecithin (a combination of phospholipids) is used in the cosmetics and food industries. Various factors such as temperature, pH, droplets size, etc. destabilize the emulsion. Therefore, the emulsion stabilizers are used to stabilize, preserve and safely deliver the formulated drugs, also as a preservative in food and stabilizer in cosmetic products. Natural emulsion stabilizers offer great advantages because they are naturally degradable, ecologically effective, non-toxic, easily available in nature, non-carcinogenic, and not harmful to health.
- emulsion stabilizer
- nanoemulsion
- emulsion technology
- biopolymer
1. Emulsion and Emulsion Stabilizer
1.1. Emulsion and Emulsification
| Properties | Emulsions | Nanoemulsions | References |
|---|---|---|---|
| Droplet size | Lager than nanoemulsions | 20–200 nm | [3][16] |
| Stability | Thermodynamically unstable | Thermodynamically stable | [4][17] |
| Formation | By high shear homogenization methods | Micro-fluidization of emulsions | [5][18] |
| Viscosity | Higher viscosity than nanoemulsions | Lower viscosity than emulsions | [5][18] |
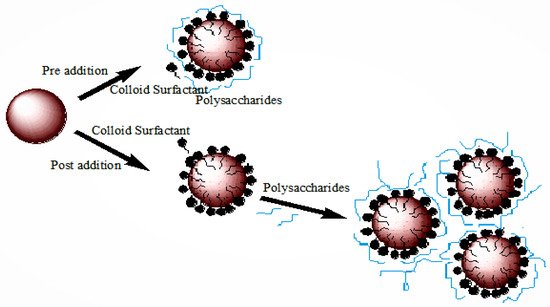
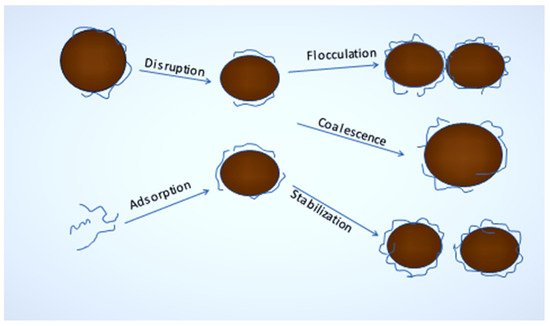
1.2. Stabilization and Destabilization of Nanoemulsion
1.3. Synthesis and Application of Nanoemulsion
| Sources | Emulsification Techniques | Droplet Size | References |
|---|---|---|---|
| Fluids | Ultrasonic emulsification | 24.21 ± 0.11 nm | [17][30] |
| Pastes | Emulsion inversion point method | <300 nm | [18][31] |
| Fogs | High-pressure homogenization | 200–600 nm | [19][32] |
| Gels | Microfluidization | <100 nm | [20][21][33,34] |
| Fine liquid and solid particles in the air | Vertex mixing | 282 nm | [22][23][35,36] |
| Topical | High-pressure homogenization | 50–100 nm | [24][37] |
| Oral | Microfluidization | 22 ± 4.0 nm | [25][38] |
| Intravenous | High-pressure homogenization | 89.23 ± 7.2 nm | [26][39] |
| Intranasal, pulmonary, and ocular | High-pressure homogenization | 8.4 ± 12.7 nm | [27][40] |
| Cosmetic industry | Ultrasonic emulsification | 6–10 nm | [28][41] |
| Pesticide industry | Low-energy emulsification | ~30 nm | [29][42] |
2. Polysaccharides Chemical Structure and Their Properties
2.1. Synthesis of Polysaccharides Emulsion
2.2. Glycosyl with Polysaccharides
3. Food Protein and Food Protein Emulsions
3.1. Animal Protein and Plant Protein
3.2. Effectiveness of Plant Protein
3.3. Pea Protein Entrapment Efficiency
3.4. Other Plant Protein and Entrapment Efficiency
3.5. Modification of Protein into Functional Components
4. The Stability Factors of Proteins Nanoemulsions
4.1. Encapsulation and Encapsulation Efficiency
4.2. Emulsifying Properties of Proteins
- (a)
-
Surface hydrophobicity: The percentage of hydrophobic proteins exposed on the surface of proteins measures how much of the protein can adsorb to the oil phase. The presence of hydrophobic sites buried inside proteins can be revealed by partial denaturation, which can increase their emulsifying ability [72][85].
- (b)
-
The flexibility of proteins: It is a self-rearrangement property of proteins, when it is adsorbed at the oil–water (O/W) interface, most of the hydrophilic mass favors the watery parts and the hydrophobic mass favors the oily parts by reducing the attractive force between two liquids [73][86]. According to the composition of protein, hydrophilic loops of amino acids may enlarge away from the O/W interface in the form of waterish parts, slowing the reaction [73][86].
- (c)
-
The dimension of the protein molecules may affect their movement on the O/W interface emulsification process, and the film formation capacities of the protein. Luyten et al. (2004) [74][87] reported that smaller proteins are more effective for diffusion at the interface than larger proteins [72][85].
- (d)
-
When encapsulating, a high solubility of proteins is preferable in order to allow higher movement in the O/W interface and higher continuous phase viscosities [72][85].
- (e)
-
The factor influences in the protein solubility are the pH of the solvent, the ionic character, and the attractive or repulsive forces between closer globules showing emulsion instability or stability. When the solvent pH is not near the isoelectric point of proteins or when ionic conditions are low, charge repulsion can enhance emulsion stability [75][88].