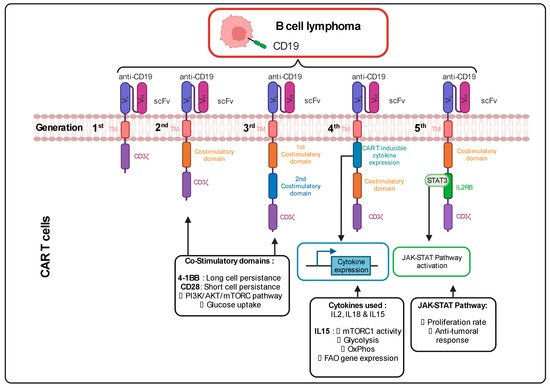
The CAR incorporated at the surface of T cells has a T cell receptor (TCR)-like structure. The extracellular part consists of a single variable chain fragment (scFv) of an antibody that recognizes the antigen present on the cancer cell surface. The transmembrane and intracellular parts contain co-stimulatory domains that permit the amplification of the signaling and, thus, the response of the T cell following binding to the tumor antigen. In the first-generation CAR T cell design, CD3ζ was the only signaling domain used, but in the following generations, CD28 and/or 4-1BB (CD137) co-stimulatory domains were added to this structure. The choice of these domains is also important for the metabolic status and the survival of the CAR T cells. It was observed in patients that the use of 4-1BB permitted a persistence of CAR T cells in time, without exhaustion of those CAR T cells, via the stimulation of the noncanonical nuclear factor kappa B (NF-κB) pathway
[1][69]. In contrast, the CD28 costimulatory domain does not permit the cells to survive longer than 30 days in the patients
[2][3][4][5][70,71,72,73]. This phenomenon can be explained by the fact that the 4-1BB promotes improved mitochondrial function so that T cells can rely on mitochondrial respiration as their energy source, which promotes the survival of central memory T cells by the activation of adenosine monophosphate kinase (AMPK), as demonstrated in mice
[6][74]. When the CD28 co-stimulatory domain was included in the CAR, patient T cells were relying more on glycolysis (via activation of the PI3K/AKT/mTOR axis), which pushed their differentiation towards effector memory T cells
[7][8][75,76]. Following antigen recognition by the CAR, CD28 stimulated GLUT1 via the PI3K/AKT pathway, linked to the mTOR/Myc pathways, which are also implicated in amino acid/lipid metabolism
[9][10][17,19]. These data underline the importance of the choice of the co-stimulatory domain to shape CAR T cell metabolism and permit short-term or long-term anti-tumor efficacy. As for CAR T cells, TIL performance also depends strongly on the metabolic status of the malignancy and the TME.
Cytokines also play a role in the metabolic shape of the CAR T cells. Indeed, the fourth CAR T cell generation, also known as “TRUCKS” (T-cell redirected for unrestricted cytokine-mediated killing), has a transgenic cytokine expression system that improves their expansion and persistence in vivo via metabolic changes
[11][77]. The cytokines employed in this strategy are interleukin 12 (IL12), IL15 and IL18. IL15 can, for example, lower the glycolysis level in human CAR T cells via a decrease in mTOR complex 1 (mTORC1) activity, whilst OXPHOS levels/respiratory capacity and expression of fatty acid oxidation-related genes are increased in these cells. This metabolic phenotype allows the human CAR T cells to have a stem cell memory behavior with higher cell proliferation and in vivo longevity
[12][78]. More recent fifth-generation CAR T cells express a sub-unit of the IL2 receptor (IL2RB) between the stimulatory domains (CD3ζ and CD28/4-1BB). Since IL2RB presents a binding site for STAT3, the cells can activate the JAK-STAT pathway, following the antigen recognition, which leads to a higher proliferation rate. Moreover, these human CAR T cells seem to have a stronger anti-cancer activity against leukemic cells
[13][79] (Figure 12).
Figure 12. Different generations of CAR T cells and their signaling pathways. TM: Transmembrane; scFv: Single-chain variable Fragment; VL: Variable Light chain; VH: Variable Heavy chain; IL2RB: interleukin 2 Receptor B; mTORC: mammalian target of rapamycin complex; FAO: Fatty Acid Oxidation; JAK-STAT: Janus Kinase-Signal Transducers and Activators of Transcription; PI3K: Phosphoinositide 3-kinase; Akt: Protein kinase; CART: Chimeric Antigen Receptor T cell. Figure generated with Biorender.