Residential and commercial buildings are responsible for over 30% of global final energy consumption and accounts for ~40% of annual direct and indirect greenhouse gas emissions. Energy efficient and sustainable technologies are necessary to not only lower the energy footprint but also lower the environmental burden. Many proven and emerging technologies are being pursued to meet the ever-increasing energy demand. Catalytic science has a significant new role to play in helping address sustainable energy challenges, particularly in buildings, compared to transportation and industrial sectors. Thermally driven heat pumps, dehumidification, cogeneration, thermal energy storage, carbon capture and utilization, emissions suppression, waste-to-energy conversion, and corrosion prevention technologies can tap into the advantages of catalytic science in realizing the full potential of such approaches, quickly, efficiently, and reliably. Catalysts can help increase energy conversion efficiency in building related technologies but must utilize low cost, easily available and easy-to-manufacture materials for large scale deployment. This entry presents a comprehensive overview of the impact of each building technology area on energy demand and environmental burden, state-of-the-art of catalytic solutions, research, and development opportunities for catalysis in building technologies, while identifying requirements, opportunities, and challenges.
- catalysis
- buildings
- heat pumps
- dehumidification
- carbon capture
- emissions
- indoor air quality
- cogeneration
- non-precious metals
- photo-catalysis
- electrocatalysis
1. Introduction
Energy plays a vital role in modern society; however, it is also responsible for greenhouse gas emissions. Global energy consumption is on the rise driven by economic and population growth. As shown in Figure 1, although renewables are expected to become the primary resource, fossil fuels continue to increase, given the global demand increase [1].
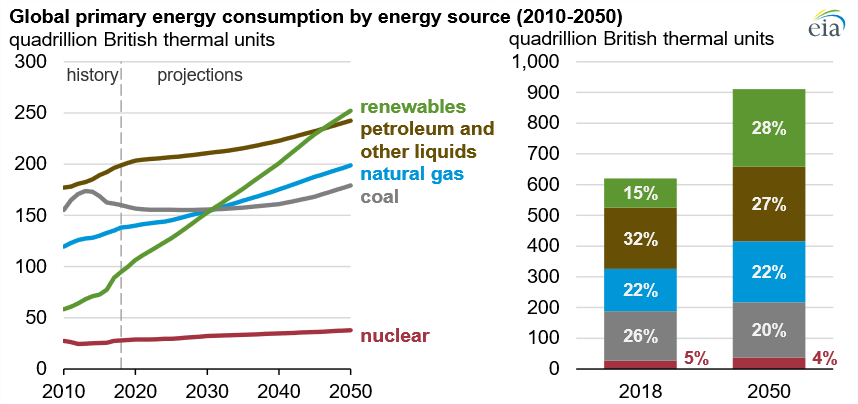
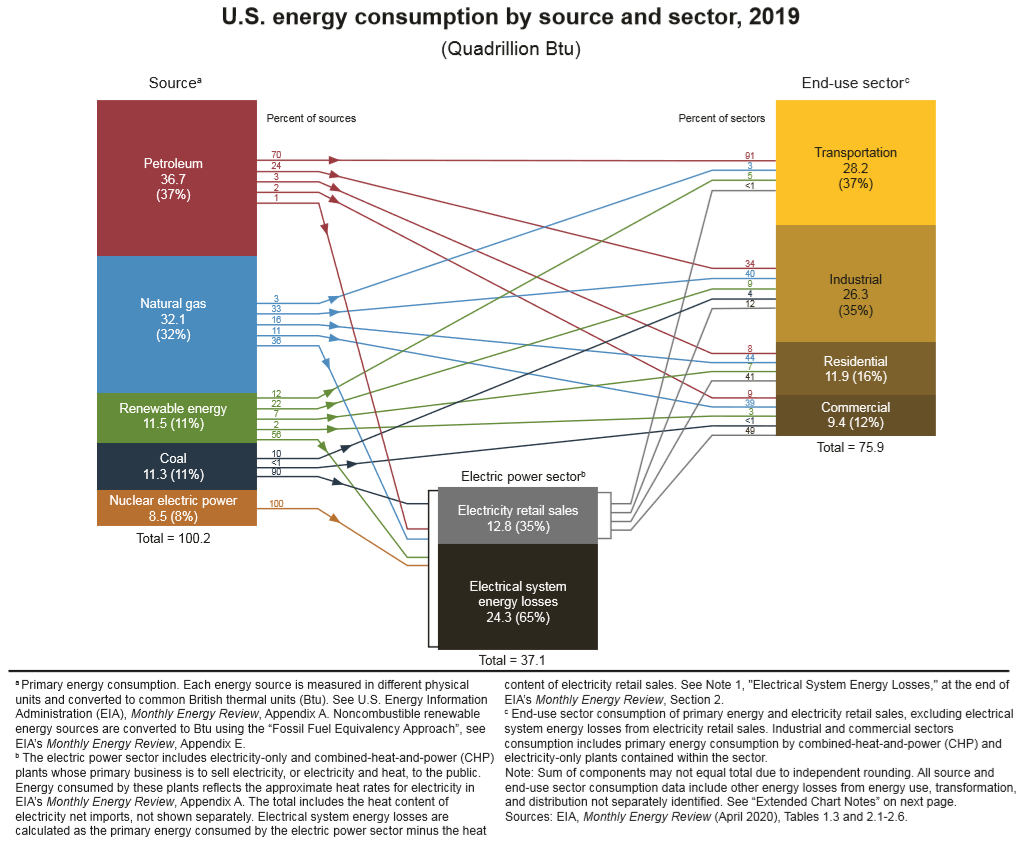
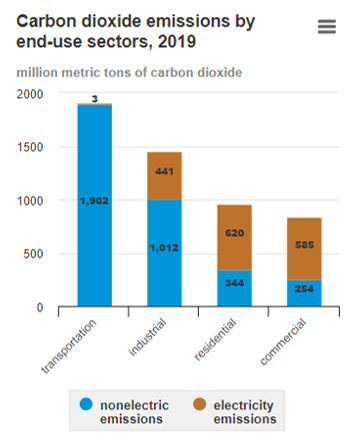
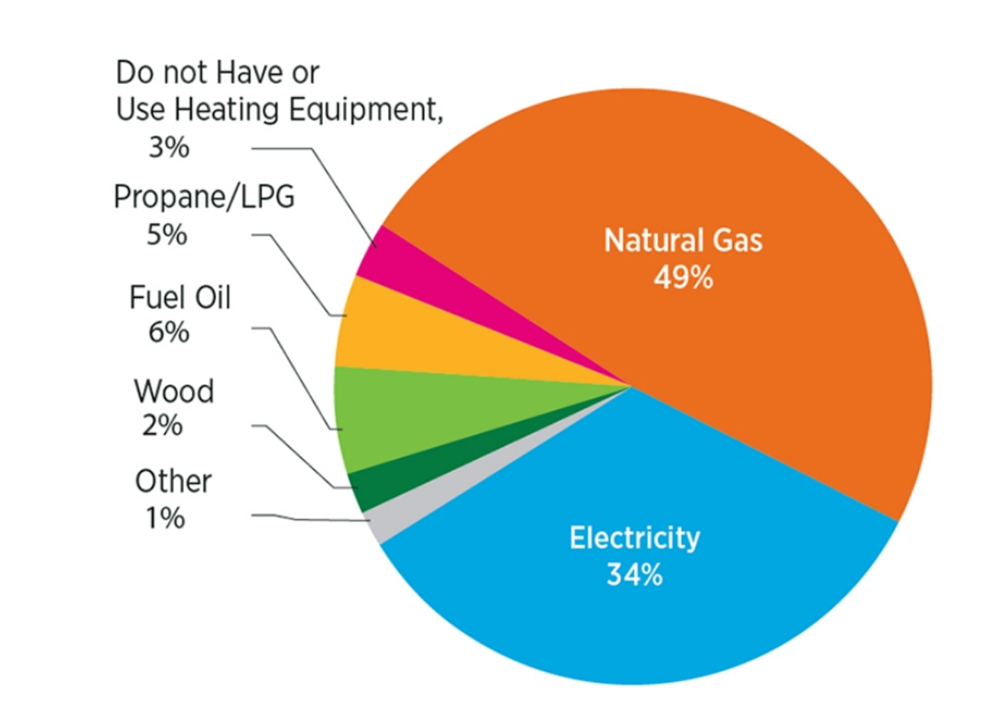 Figure 3. U.S. household heating systems’ energy source [7].
Figure 3. U.S. household heating systems’ energy source [7].
2. Catalysts in Building Technologies
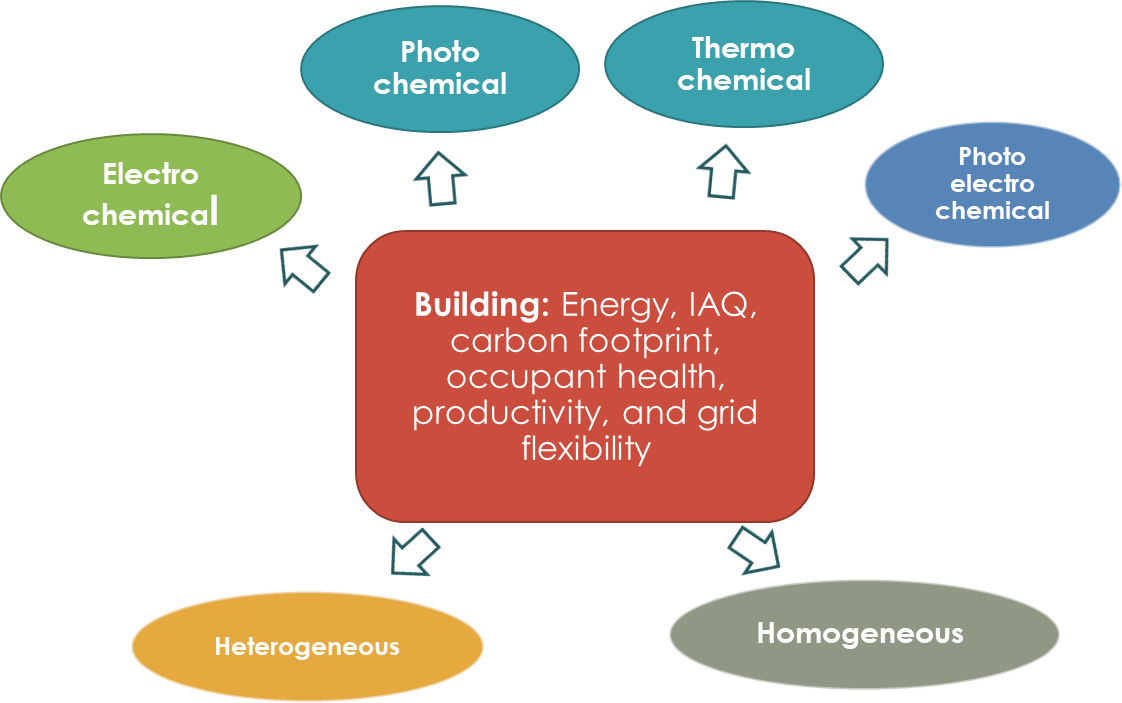
As discussed above, energy and environmental challenges associated with buildings can be overcome through the application of catalytic science. Many energy consumers within building envelope can exploit the advantages offered by innovations in the field of catalysis. Application of various catalytic reaction schemes shown in Figure 5 include electrochemical, photochemical, photoelectrochemical, and thermochemical can aid in lowering the environmental and energy burden of the buildings. The rest of this section provides an overview of this.
2.1. Indoor Air Quality and Emissions
2.2. Dehumidification
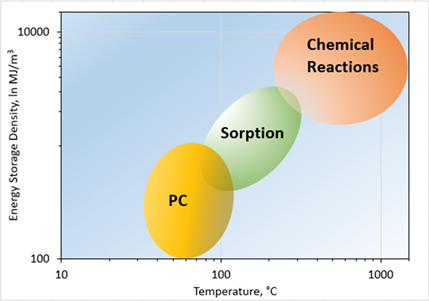
2.3. Thermal Energy Storage
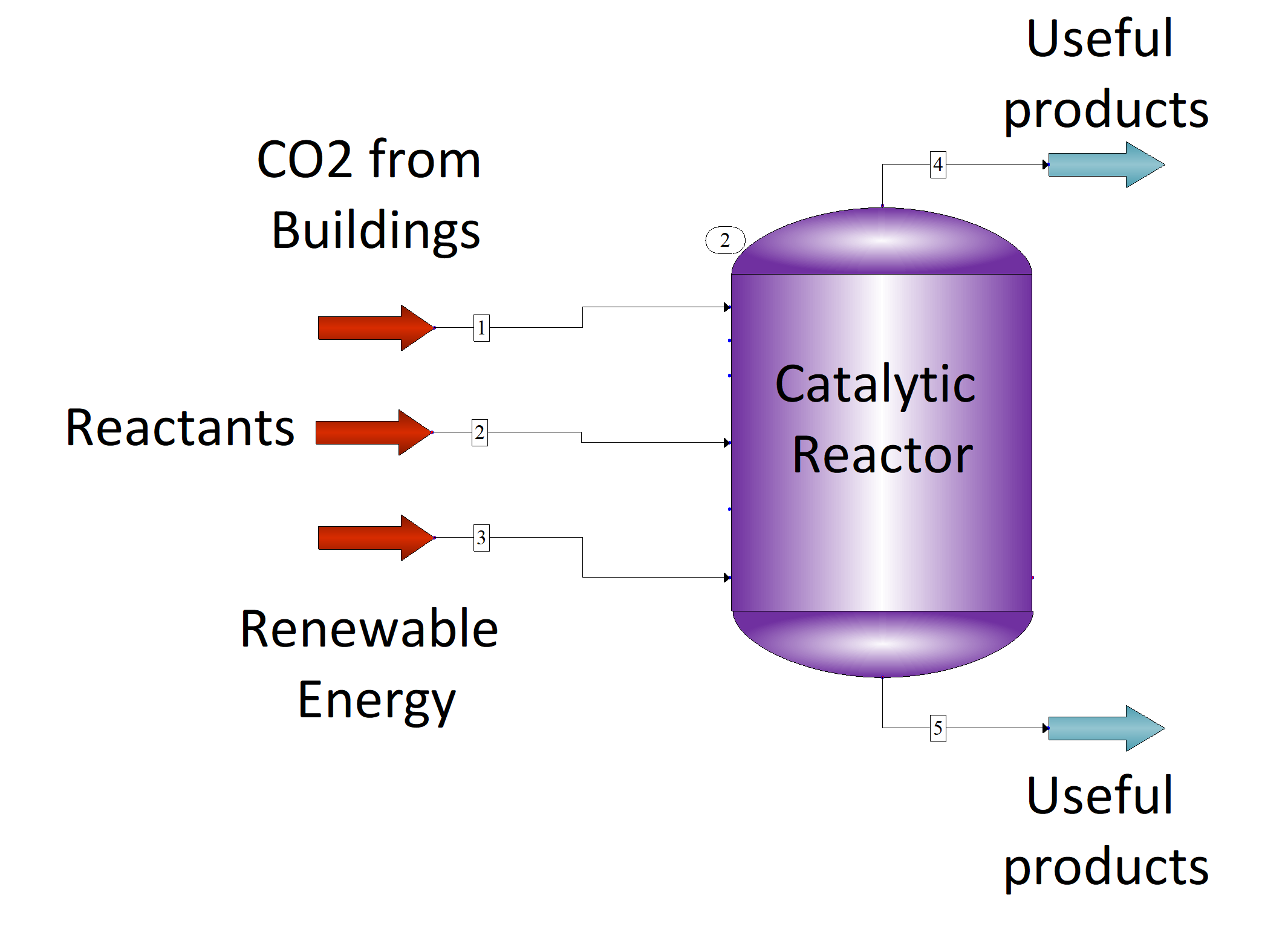
2.4. Carbon Dioxide Capture and Conversion
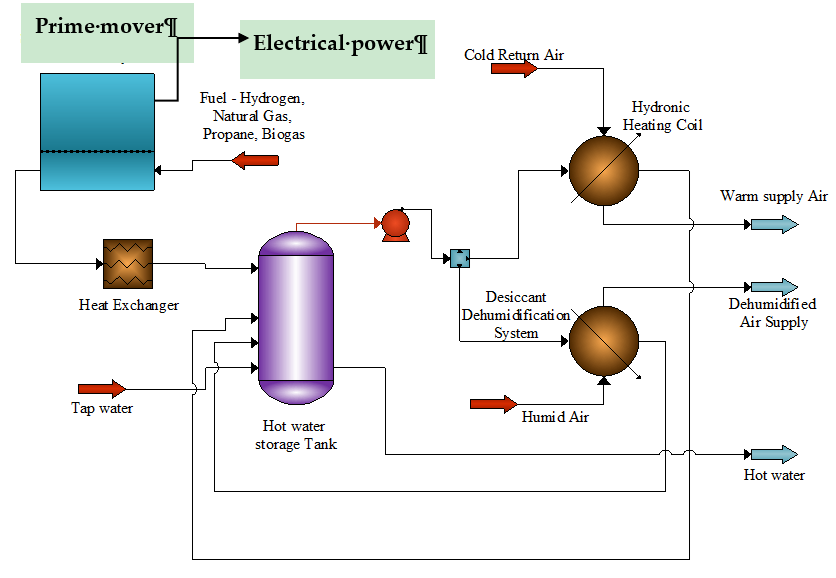
2.5. Heating and Cooling

2.6. Cogeneration
3. Discussion
Nomenclature & Abbreviations
| Btu | British Thermal Units |
| EJ | Exajoules |
| g | gram |
| h | Hour |
| kg | Kilogram |
| kJ | Kilo Joules |
| mL | Milliliter |
| MJ | Mega Joules |
| TWh | Terawatt-hours |
| CNT | Carbon Nano Tubes |
| EIA | Energy Information Administration |
| EPA | Environment Protection Agency |
| HVAC | Heating, Ventilation, and Air Conditioning |
| IAQ | Indoor Air Quality |
| IEA | International Energy Agency |
| MOF | Metal Oxide Framework |
| NIH | National Institute of Health |
| OER | Oxygen Evolution Reaction |
| ORR | Oxygen Reduction Reaction |
| Quad | Quadrillion |
| PEM | Polymer Electrolyte Fuel Cells |
| PGM | Precious Group Metals |
| RH | Relative Humidity |
| RTIL | Room Temperature Ionic Liquids |
| SAPO | Silicoalumino Phosphate Zeolite |
| SOFC | Solid Oxide Fuel Cell |
| USA | United States of America |
| VOC | Volatile Organic Compounds |
References
- U.S. Energy Information Administration. EIA Projects Nearly 50% Increase in World Energy Use by 2050, Led by Growth in Renewables. Available online: https://www.eia.gov/todayinenergy/detail.php?id=49876 (accessed on 21 December 2021).
- U.S. Energy Information Administration. Global Primary Energy Consumption by Energy Source (2010–2050). Available online: https://www.eia.gov/todayinenergy/detail.php?id=41433 (accessed on 21 December 2021).
- International Energy Agency. Tracking Buildings. Available online: https://www.iea.org/reports/tracking-buildings-2020 (accessed on 21 December 2021).
- U.S. Energy Information Administration. How the United States Uses Energy. Available online: https://www.eia.gov/energyexplained/use-of-energy/ (accessed on 30 September 2021).
- U.S. Energy Information Administration. Residential Energy Consumption Survey (RECS). Available online: https://www.eia.gov/consumption/residential/ (accessed on 30 September 2021).
- U.S. Energy Information Administration. Commercial Buildings Energy Consumption Survey (CBECS). Available online: https://www.eia.gov/consumption/commercial/reports/ (accessed on 31 January 2021).
- U.S. Department of Energy. Home Heating Systems. Available online: https://www.energy.gov/energysaver/heat-and-cool/home-heating-systems (accessed on 28 February 2021).
- U.S. Energy Information Administration. Energy and the Environment Explained, Where Greenhouse Gases Come from. Available online: https://www.eia.gov/energyexplained/energy-and-the-environment/where-greenhouse-gases-come-from.php (accessed on 31 January 2021).
- Allen, J.G.; MacNaughton, P.; Satish, U.; Santanam, S.; Vallarino, J.; Spengler, J.D. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: A controlled exposure study of green and conventional office environments. Environ. Health Perspect. 2016, 124, 805–812.
- Huang, K.; Sun, W.; Feng, G.; Wang, J.; Song, J. Indoor air quality analysis of 8 mechanically ventilated residential buildings in northeast China based on long-term monitoring. Sustain. Cities Soc. 2020, 54, 101947.
- Spiru, P.; Simona, P.L. A review on interactions between energy performance of the buildings, outdoor air pollution and the indoor air quality. Energy Procedia 2017, 128, 179–186.
- Ma, N.; Aviv, D.; Guo, H.; Braham, W.W. Measuring the right factors: A review of variables and models for thermal comfort and indoor air quality. Renew. Sustain. Energy Rev. 2021, 135, 110436.
- Cheekatamarla, P. Performance analysis of hybrid power configurations: Impact on primary energy intensity, carbon dioxide emissions, and life cycle costs. Int. J. Hydrog. Energy 2020, 45, 34089–34098.
- Cheekatamarla, P.; Abu-Heiba, A. A Comprehensive Review and Qualitative Analysis of Micro-Combined Heat and Power Modeling Approaches. Energies 2020, 13, 3581.
- Sun, D.; Le, Y.; Jiang, C.; Cheng, B. Ultrathin Bi2WO6 nanosheet decorated with Pt nanoparticles for efficient formaldehyde removal at room temperature. Appl. Surf. Sci. 2018, 441, 429–437.
- Wallace, L.A. Total Exposure Assessment Methodology (TEAM) Study: Summary and Analysis; Environmental Protection Agency: Washington, DC, USA, 1987; Volume 1.
- Abdul-Wahab, S.A.; En, S.C.F.; Elkamel, A.; Ahmadi, L.; Yetilmezsoy, K. A review of standards and guidelines set by international bodies for the parameters of indoor air quality. Atmos. Pollut. Res. 2015, 6, 751–767.
- World Health Organization. WHO Guildelines for Indoor Air Quality. Available online: https://www.euro.who.int/__data/assets/pdf_file/0009/128169/e94535.pdf (accessed on 31 January 2021).
- Yue, X.; Ma, N.L.; Sonne, C.; Guan, R.; Lam, S.S.; Van Le, Q.; Chen, X.; Yang, Y.; Gu, H.; Rinklebe, J. Mitigation of indoor air pollution: A review of recent advances in adsorption materials and catalytic oxidation. J. Hazard. Mater. 2020, 405, 124138.
- Ibáñez Gómez, J.A.; Giampiccolo, A.; Tobaldi, D.M.; Mair, S.; da Silva, C.F.; Barrasa, A.M.C.; Maskell, D.; Ansell, M.P.; Kurchania, R.; Mayer, F. Photocatalytic lime render for indoor and outdoor air quality improvement. Catalysts 2021, 11, 296.
- Giosuè, C.; Pierpaoli, M.; Mobili, A.; Ruello, M.L.; Tittarelli, F. Influence of binders and lightweight aggregates on the properties of cementitious mortars: From traditional requirements to indoor air quality improvement. Materials 2017, 10, 978.
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase–A review. Chem. Eng. J. 2018, 334, 2408–2439.
- Rezaee, A.; Rangkooy, H.; Khavanin, A.; Jafari, A.J. High photocatalytic decomposition of the air pollutant formaldehyde using nano-ZnO on bone char. Environ. Chem. Lett. 2014, 12, 353–357.
- Li, Y.; Zhang, C.; Ma, J.; Chen, M.; Deng, H.; He, H. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation. Appl. Catal. B Environ. 2017, 217, 560–569.
- Li, X.; Qian, X.; An, X.; Huang, J. Preparation of a novel composite comprising biochar skeleton and “chrysanthemum” g-C3N4 for enhanced visible light photocatalytic degradation of formaldehyde. Appl. Surf. Sci. 2019, 487, 1262–1270.
- Nasriddinov, A.; Rumyantseva, M.; Marikutsa, A.; Gaskov, A.; Lee, J.-H.; Kim, J.-H.; Kim, J.-Y.; Kim, S.S.; Kim, H.W. Sub-ppm formaldehyde detection by nn TiO2@ SnO2 nanocomposites. Sensors 2019, 19, 3182.
- Zeng, Y.; Xie, R.; Cao, J.; Chen, Z.; Fan, Q.; Liu, B.; Lian, X.; Huang, H. Simultaneous removal of multiple indoor-air pollutants using a combined process of electrostatic precipitation and catalytic decomposition. Chem. Eng. J. 2020, 388, 124219.
- Zhao, G.; Zou, J.; Zhang, T.; Li, C.; Zhou, S.; Jiao, F. Recent progress on removal of indoor air pollutants by catalytic oxidation. Rev. Environ. Health 2020, 35, 311–321.
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559.
- Malayeri, M.; Haghighat, F.; Lee, C.-S. Modeling of volatile organic compounds degradation by photocatalytic oxidation reactor in indoor air: A review. Build. Environ. 2019, 154, 309–323.
- Nath, R.K.; Zain, M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194.
- Zang, M.; Zhao, C.; Wang, Y.; Chen, S. A review of recent advances in catalytic combustion of VOCs on perovskite-type catalysts. J. Saudi Chem. Soc. 2019, 23, 645–654.
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453.
- Guo, J.; Lin, C.; Jiang, C.; Zhang, P. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255.
- Singer, B.C.; Pass, R.Z.; Delp, W.W.; Lorenzetti, D.M.; Maddalena, R.L. Pollutant concentrations and emission rates from natural gas cooking burners without and with range hood exhaust in nine California homes. Build. Environ. 2017, 122, 215–229.
- U.S. Energy Information Administration. Why and Where Mold Grows? Available online: https://www.epa.gov/mold/mold-course-chapter-2 (accessed on 28 February 2021).
- Yang, W.; Marr, L.C. Mechanisms by Which Ambient Humidity May Affect Viruses in Aerosols. Appl Environ Microbiol. 2012, 78, 6781–6788.
- Vellei, M.; Herrera, M.; Fosas, D.; Natarajan, S. The influence of relative humidity on adaptive thermal comfort. Build. Environ. 2017, 124, 171–185.
- Zonno, G.; Aguilar, R.; Boroschek, R.; Lourenço, P.B. Analysis of the long and short-term effects of temperature and humidity on the structural properties of adobe buildings using continuous monitoring. Eng. Struct. 2019, 196, 109299.
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Zheng, Y.-R.; Yu, S.-H. Water oxidation electrocatalyzed by an efficient Mn3O4/CoSe2 nanocomposite. J. Am. Chem. Soc. 2012, 134, 2930–2933.
- Liu, G.; Xu, J.; Wang, Y.; Wang, X. An oxygen evolution catalyst on an antimony doped tin oxide nanowire structured support for proton exchange membrane liquid water electrolysis. J. Mater. Chem. A 2015, 3, 20791–20800.
- Oh, H.S.; Nong, H.N.; Strasser, P. Preparation of mesoporous Sb-, F-, and In-doped SnO2 bulk powder with high surface area for use as catalyst supports in electrolytic cells. Adv. Funct. Mater. 2015, 25, 1074–1081.
- Li, D.; Yan, W.; Qi, R.; Zhang, L.-Z. Development of structurally modified OER catalysts with enhanced performance and longevity for PEM-based electrolytic air dehumidification. Int. J. Hydrog. Energy 2021, 46, 9267–9279.
- Zhou, G.; Shan, Y.; Hu, Y.; Xu, X.; Long, L.; Zhang, J.; Dai, J.; Guo, J.; Shen, J.; Li, S. Half-metallic carbon nitride nanosheets with micro grid mode resonance structure for efficient photocatalytic hydrogen evolution. Nat. Commun. 2018, 9, 1–9.
- Wang, Z.; Inoue, Y.; Hisatomi, T.; Ishikawa, R.; Wang, Q.; Takata, T.; Chen, S.; Shibata, N.; Ikuhara, Y.; Domen, K. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat. Catal. 2018, 1, 756–763.
- Yang, L.; Ravi, S.K.; Nandakumar, D.K.; Alzakia, F.I.; Lu, W.; Zhang, Y.; Yang, J.; Zhang, Q.; Zhang, X.; Tan, S.C. A hybrid artificial photocatalysis system splits atmospheric water for simultaneous dehumidification and power generation. Adv. Mater. 2019, 31, 1902963.
- Fang, Y.; Liu, T.; Zhang, Z.; Gao, X. Silica gel adsorbents doped with Al, Ti, and Co ions improved adsorption capacity, thermal stability and aging resistance. Renew. Energy 2014, 63, 755–761.
- Aristov, Y.I.; Tokarev, M.; Gordeeva, L.; Snytnikov, V.; Parmon, V. New composite sorbents for solar-driven technology of fresh water production from the atmosphere. Sol. Energy 1999, 66, 165–168.
- Ge, T.; Ziegler, F.; Wang, R. A mathematical model for predicting the performance of a compound desiccant wheel (A model of compound desiccant wheel). Appl. Therm. Eng. 2010, 30, 1005–1015.
- Zhang, X.; Sumathy, K.; Dai, Y.; Wang, R. Dynamic hygroscopic effect of the composite material used in desiccant rotary wheel. Sol. Energy 2006, 80, 1058–1061.
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715.
- Bauer, T.; Steinmann, W.-D.; Laing, D.; Tamme, R. Thermal energy storage materials and systems. Annu. Rev. Heat Transf. 2012, 15, 131–177.
- Prasad, J.S.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733.
- Manickam, K.; Mistry, P.; Walker, G.; Grant, D.; Buckley, C.E.; Humphries, T.D.; Paskevicius, M.; Jensen, T.; Albert, R.; Peinecke, K. Future perspectives of thermal energy storage with metal hydrides. Int. J. Hydrog. Energy 2019, 44, 7738–7745.
- Mistry, P.C.; Grant, D.M.; Stuart, A.D.; Manickam, K.; Walker, G.S. Evolution of catalyst coated atomised magnesium spheres–An alternative thermal storage medium for concentrated solar power applications. Int. J. Hydrog. Energy 2017, 42, 28453–28463.
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253, 1–9.
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585.
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193.
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26.
- Du, J.; Yang, X.-x.; Ding, J.; Wei, X.-l.; Yang, J.-p.; Wang, W.-l.; Yang, M.-l. Carbon dioxide reforming of methane over bimetallic catalysts of Pt-Ru/γ-Al 2 O 3 for thermochemical energy storage. J. Cent. South Univ. 2013, 20, 1307–1313.
- Tsongidis, N.I.; Karagiannakis, G.; Sakellariou, K.G.; Pagkoura, C.; Konstandopoulos, A.G. Iron oxide-based particles for high temperature thermochemical energy storage via the elemental sulfur thermochemical cycle. In Proceedings of AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2019; p. 210009.
- Yang, L.; Huang, Z.; Huang, G. Fe-and Mn-Doped Ca-Based Materials for Thermochemical Energy Storage Systems. Energy Fuels 2020, 34, 11479–11488.
- Samanta, A.; Zhao, A.; Shimizu, G.K.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463.
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090.
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441.
- Li, B.; Duan, Y.; Luebke, D.; Morreale, B. Advances in CO2 capture technology: A patent review. Appl. Energy 2013, 102, 1439–1447.
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525.
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681.
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289.
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151.
- Lee, S.-Y.; Park, S.-J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11.
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013–A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148.
- Prasetya, N.; Himma, N.F.; Sutrisna, P.D.; Wenten, I.G.; Ladewig, B.P. A review on emerging organic-containing microporous material membranes for carbon capture and separation. Chem. Eng. J. 2019, 391, 123575.
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63.
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-doped graphitic carbon nitride nanotubes with amino-rich surface for efficient CO2 capture, enhanced photocatalytic activity, and product selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009.
- Xie, B.; Lovell, E.; Tan, T.H.; Jantarang, S.; Yu, M.; Scott, J.; Amal, R. Emerging material engineering strategies for amplifying photothermal heterogeneous CO2 catalysis. J. Energy Chem. 2020, 59, 108–125.
- Wang, L.; Dong, Y.; Yan, T.; Hu, Z.; Jelle, A.A.; Meira, D.M.; Duchesne, P.N.; Loh, J.Y.Y.; Qiu, C.; Storey, E.E. Black indium oxide a photothermal CO2 hydrogenation catalyst. Nat. Commun. 2020, 11, 1–8.
- Jantarang, S.; Lovell, E.C.; Tan, T.H.; Scott, J.; Amal, R. Role of support in photothermal carbon dioxide hydrogenation catalysed by Ni/CexTiyO2. Prog. Nat. Sci. Mater. Int. 2018, 28, 168–177.
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185.
- Jones, J.P.; Prakash, G.S.; Olah, G.A. Electrochemical CO2 reduction: Recent advances and current trends. Isr. J. Chem. 2014, 54, 1451–1466.
- Kumar, S.; Wani, M.Y.; Arranja, C.T.; e Silva, J.d.A.; Avula, B.; Sobral, A.J. Porphyrins as nanoreactors in the carbon dioxide capture and conversion: A review. J. Mater. Chem. A 2015, 3, 19615–19637.
- Lee, M.-Y.; Park, K.T.; Lee, W.; Lim, H.; Kwon, Y.; Kang, S. Current achievements and the future direction of electrochemical CO2 reduction: A short review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 769–815.
- Lim, H.-K.; Kim, H. The mechanism of room-temperature ionic-liquid-based electrochemical CO2 reduction: A review. Molecules 2017, 22, 536.
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 zeolite filled macrocellular foams for adsorption heat pump applications: A preliminary study. Appl. Therm. Eng. 2017, 124, 1312–1318.
- Restuccia, G.; Recupero, V.; Cacciola, G.; Rothmeyer, M. Zeolite heat pump for domestic heating. Energy 1988, 13, 333–342.
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Schossig, P.; Henning, H.-M. Novel sorption materials for solar heating and cooling. Energy Procedia 2012, 30, 279–288.
- Mandal, J.; Fu, Y.; Overvig, A.C.; Jia, M.; Sun, K.; Shi, N.N.; Zhou, H.; Xiao, X.; Yu, N.; Yang, Y. Hierarchically porous polymer coatings for highly efficient passive daytime radiative cooling. Science 2018, 362, 315–319.
- Cui, S.; Marandi, A.; Lebourleux, G.; Thimon, M.; Bourdon, M.; Chen, C.; Severino, M.I.; Steggles, V.; Nouar, F.; Serre, C. Heat properties of a hydrophilic carboxylate-based MOF for water adsorption applications. Appl. Therm. Eng. 2019, 161, 114135.
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A review of chemical heat pump technology and applications. Appl. Therm. Eng. 2001, 21, 1489–1519.
- Xin, F.; Xu, M.; Huai, X.-L.; Li, X.-F. Characteristic and kinetic of liquid-phase isopropanol dehydrogenation over Raney nickel catalysts for chemical heat pump. Appl. Therm. Eng. 2014, 70, 580–585.
- Cai, J.; Li, X.; Tao, Y.; Huai, X.; Guo, Z. Advances in Organic Liquid-Gas Chemical Heat Pumps. Chem. Eng. Technol. 2011, 34, 1603–1613.
- Cheekatamarla, P.K.; Finnerty, C. Reforming catalysts for hydrogen generation in fuel cell applications. J. Power Sources 2006, 160, 490–499.
- Xu, X.; Shuai, K.; Xu, B. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen. Catalysts 2017, 7, 183.
- Bepari, S.; Kuila, D. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts—A review. Int. J. Hydrog. Energy 2020, 45, 18090–18113.
- Ogo, S.; Sekine, Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: A review. Fuel Process. Technol. 2020, 199, 106238.
- Chen, J.; Sun, J.; Wang, Y. Catalysts for steam reforming of bio-oil: A review. Ind. Eng. Chem. Res. 2017, 56, 4627–4637.
- Aziz, M.; Setiabudi, H.; Teh, L.; Annuar, N.; Jalil, A. A review of heterogeneous catalysts for syngas production via dry reforming. J. Taiwan Inst. Chem. Eng. 2019, 101, 139–158.
- Setiabudi, H.; Aziz, M.; Abdullah, S.; Teh, L.; Jusoh, R. Hydrogen production from catalytic steam reforming of biomass pyrolysis oil or bio-oil derivatives: A review. Int. J. Hydrog. Energy 2020, 45, 18376–18397.
- Arku, P.; Regmi, B.; Dutta, A. A review of catalytic partial oxidation of fossil fuels and biofuels: Recent advances in catalyst development and kinetic modelling. Chem. Eng. Res. Des. 2018, 136, 385–402.
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352.
- Lehtoranta, K.; Murtonen, T.; Vesala, H.; Koponen, P.; Alanen, J.; Simonen, P.; Rönkkö, T.; Timonen, H.; Saarikoski, S.; Maunula, T. Natural gas engine emission reduction by catalysts. Emiss. Control Sci. Technol. 2017, 3, 142–152.
- Hutter, R.; De Libero, L.; Elbert, P.; Onder, C.H. Catalytic methane oxidation in the exhaust gas aftertreatment of a lean-burn natural gas engine. Chem. Eng. J. 2018, 349, 156–167.
- Silas, K.; Ghani, W.A.W.A.K.; Choong, T.S.; Rashid, U. Carbonaceous materials modified catalysts for simultaneous SO2/NOx removal from flue gas: A review. Catal. Rev. 2019, 61, 134–161.
- Gholami, Z.; Luo, G.; Gholami, F.; Yang, F. Recent advances in selective catalytic reduction of NOx by carbon monoxide for flue gas cleaning process: A review. Catal. Rev. 2020, 63, 68–119.
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-j. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825.
- Ioroi, T.; Siroma, Z.; Yamazaki, S.i.; Yasuda, K. Electrocatalysts for PEM fuel cells. Adv. Energy Mater. 2019, 9, 1801284.
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-based metal-free ORR electrocatalysts for fuel cells: Past, present, and future. Adv. Mater. 2019, 31, 1804799.
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30.
- Ercolano, G.; Cavaliere, S.; Roziere, J.; Jones, D.J. Recent developments in electrocatalyst design thrifting noble metals in fuel cells. Curr. Opin. Electrochem. 2018, 9, 271–277.
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 1–15.
- Qiu, P.; Yang, X.; Zhu, T.; Sun, S.; Jia, L.; Li, J. Review on core-shell structured cathode for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2020, 45, 23160–23173.
- Abd Aziz, A.J.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications. Ceram. Int. 2020, 46, 23314–23325.
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden-Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 022002.
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review. Prog. Energy Combust. Sci. 2017, 61, 57–77.
- Niakolas, D.K.; Neofytidis, C.S.; Neophytides, S.G. Effect of au and/or Mo doping on the development of carbon and sulfur tolerant anodes for SOFCs—A short review. Front. Environ. Sci. 2017, 5, 78.
- Cheekatamarla, P.K.; Finnerty, C.M.; Du, Y.; Jiang, J.; Dong, J.; Dewald, P.; Robinson, C. Advanced tubular solid oxide fuel cells with high efficiency for internal reforming of hydrocarbon fuels. J. Power Sources 2009, 188, 521–526.
- Cheekatamarla, P. Performance and Reliability Advancements in a Durable Low Temperature Tubular SOFC; Atrex Energy Inc.: Walpole, MA, USA, 2019.
- Lyu, Z.; Li, H.; Han, M. Electrochemical properties and thermal neutral state of solid oxide fuel cells with direct internal reforming of methane. Int. J. Hydrog. Energy 2019, 44, 12151–12162.
- Shabri, H.A.; Othman, M.H.D.; Mohamed, M.A.; Kurniawan, T.A.; Jamil, S.M. Recent progress in metal-ceramic anode of solid oxide fuel cell for direct hydrocarbon fuel utilization: A review. Fuel Process. Technol. 2021, 212, 106626.
- Fu, H.-C.; You, F.; Li, H.-R.; He, L.-N. CO2 Capture and in situ Catalytic Transformation. Front. Chem. 2019, 7, 525.
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, Y.; Song, Y.; Li, H. Alternative pathways for efficient CO2 capture by hybrid processes—A review. Renew. Sustain. Energy Rev. 2018, 82, 215–231.
