Fungi can synthesize a wealth of secondary metabolites, which are widely used in the exploration of lead compounds of pharmaceutical or agricultural importance. Beauveria, Metarhizium, and Cordyceps are the most extensively studied fungi in which a large number of biologically active metabolites have been identified. However, relatively little attention has been paid to Purpureocillium lilacinum. P. lilacinum are soil-habituated fungi that are widely distributed in nature and are very important biocontrol fungi in agriculture, providing good biological control of plant parasitic nematodes and having a significant effect on Aphidoidea, Tetranychus cinnbarinus, and Aleyrodidae. At the same time, it produces secondary metabolites with various biological activities such as anticancer, antimicrobial, and insecticidal.
1. Introduction
The genus
Purpureocillium in the Ophiocordycipitaceae family was structured by Luangsa-Ard et al. In 2011, based on the medical importance, the
Purpureocillium lilacinum was designated as the type species of the
Paecilomyces genus
[1]. This species was nominated as
Penicillium lilacinum by Thom in 1901, and then it was revised as
Paecilomyces lilacinus by Samson in 1974
[2]. After comparing the 18S rRNA gene, internal transcribed spacer, and partial translation elongation factor 1-a sequences with
P. lilacinus, Luangsa-Ard proposed a new genus name
Purpureocillium and made the new combination
P. lilacinum in 2011. The fungus was found in a wide range of land and marine environments
[3][4][5][3,4,5]. They are often isolated from insects, nematodes, and the rhizosphere of many crops
[6][7][8][6,7,8]. The species can grow in a wide range of temperatures from 8 to 38 °C with optimal temperatures of 26–30 °C
[3]. It also has a wide pH tolerance and can grow on a variety of substrates
[9]. This fungus has promising potential as a biocontrol agent to control crops‘ root-knot nematodes
[10][11][10,11]. The parasitism of nematodes is that the hyphae directly invade the surface of nematodes’ eggs and then produce appressoria on the surface, which infects the nematodes’ eggs after adsorption. In the process of infection,
P. lilacinum secretes a variety of enzymes, such as serine protease and chitinase, which can lead to the degradation of protein and chitin components of the nematode epidermis, which is conducive to the invasion of fungi and destruction of cell components. It has been shown that the fermentation filtrate of
P. lilacinum can inhibit the mycelial growth of the pathogenic fungi
Helminthosporium maydis and
Fusarium graminearum, and has a significant inhibitory effect on the spore germination of
Fusarium oxysporum [12][13][12,13]. Currently, there are eight registered pesticide products of
P. lilacinum in China used to control root-knot nematodes (
http://www.chinapesticide.org.cn/hysj/index.jhtml, accessed on 23 February 2021); similar pesticides are also registered in the USA (
https://iaspub.epa.gov/apex/pesticides, accessed on 23 February 2021) and European Union (
http://www.efsa.europa.eu/, accessed on 23 February 2021). In addition,
P. lilacinum has been shown to be effective against
Phyllotreta striolata, Thrips palmi, and predatory mite
[14][15][14,15]. However, the
P. lilacinum strains used for biocontrol agents have a high identity with those strains causing infections in humans
[16][17][16,17].
Secondary metabolites are produced in a certain growth period of plants and microorganisms. They are small molecules with complex chemical structures that are not necessary for growth and reproduction, such as pigments, hormones, toxins, and antibiotics
[18]. Fungi are important organisms that produce active secondary metabolites. Different kinds of fungi produce different secondary metabolites. The discovery of fungal secondary metabolites has become an important source of new drugs and pesticides
[19]. Fumosorinone was isolated from the
Isaria fumosorosea, and it is a potential medicine for the treatment of type II diabetes and other associated metabolic syndromes
[20]. Diorcinol K, D, and I were isolated from
Aspergillus, displaying significant antibacterial activities against
Staphylococcus aureus and methicillin-resistant
S. aureus [21]. Pyrenocine A was produced by
Paecilomyces and showed a significant antitrypanosomal activity against
Trypanosoma brucei [22]. Paeciloxanthone was isolated from the extracts of
Paecilomyces sp. and showed significant cytotoxicity against HepG2 cell lines
[23]. The research on the synthesis and regulation of secondary metabolites is helpful to develop new active compounds and increase the output of active compounds. The common secondary metabolites of fungi are polyketones, nonribosome peptides, sterols, alkaloids, and terpenes.
2. Biosynthesis of Secondary Metabolites in Purpureocillium lilacinum
In 2015, Prasad sequenced the TERIBC-1 strain of
P. lilacinus with a genome size of 40.02 Mb by using Illumina Hiseq technology, and predicted 30 secondary metabolite synthesis genes: 12 polyketide syntheses (PKs, details of all abbreviations are in
Table S1), 2 PKs-like, 7 nonribosome peptide synthetases, 7 NRPSs-like, 1 PK-NRPS, and 1 dimethylallyl tryptophan synthases (DMATs) gene
[24][67]. In 2016, Wang sequenced
P. lilacinus PLBJ-1 and PLFJ-1 strains. The genome sizes of the two strains were 38.14 Mb and 38.53 Mb, respectively
[18]. Using SMURF
[25][68] and anti-SMASH
[26][69] software to predict the secondary metabolite synthesis gene cluster, PLBJ-1 and PLFJ-1 strains were found to encode 13 PKs, 2 PKs-like, 10 NRPSs, 10 NRPSs-like, 1 PK-NRPS, 4 terpene synthases (TSs), and 1 DMAT genes. It can be seen that the secondary metabolites produced by different species of
P. lilacinum are not identical, but in general,
P. lilacinum has great potential in the synthesis of secondary metabolites.
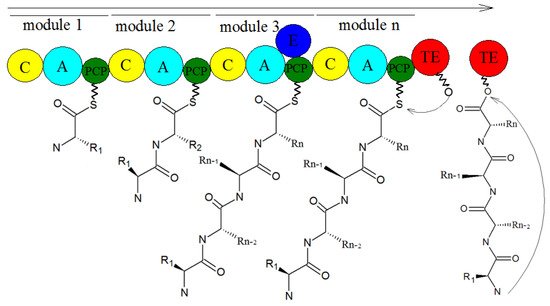
We know that the typical NRPS modules have adenylation (A), thiolation (T) or peptidyl carrier (PCP), condensation (C), and thioesterase (TE) domains
[27][28][70,71], which are, respectively, responsible for the activation of amino acids, the extension of peptide chains, the formation of amide bonds, and the release of peptide chains
[29][30][72,73]. The synthesis mechanism of NRPSs is shown in
Figure 11. Generally speaking, the A domain combines with the amino acid substrate under the action of ATP to form the corresponding aminoacyl AMP, and the aminoacyl AMP combines with the sulfhydryl group of the T domain to form the aminoacyl-s-carrier complex. Finally, the carriers carrying the aminoacyl group and the peptide acyl group combine with the specific region of the C domain, and the amino group on the aminoacyl-s-carrier complex attacks the acyl group on the peptidyl-s-carrier complex, forming a new peptide bond, and finally forming a complete peptide chain, through the action of multiple modules, wherein the amino acids in the peptide chain correspond to the modules in the NRPS one by one. Some NRPS modules also contain epimerization (E), formylation (F), methylation (M), heterocyclization (CY)
[31][74], reduction (R), and oxidation (OX) domains, which are involved in the structural modification of peptide chains. Finally, mature peptide chains are released from the NRP assembly line under the action of the TE domain
[32][75].
Figure 11. The biosynthesis of NRPSs
[33][76].
In 2016, Wang sequenced and analyzed the whole genome of
P. lilacinum, and predicted the knock-out of the NRPS synthetic gene (LcsA), PK synthetase (LcsC), Acyl CoA ligase (LcsD), and thioesterase (LcsE), using high-performance liquid chromatography (HPLC) to compare the crude extracts of wild-type and mutant strains of
P. lilacinum. It was found that the crude extracts of ΔLcsA, ΔLcsC, ΔLcsD, and ΔLcsE had a lack of Leucinostatin A and Leucinostatin B, and then these enzymes were found to play a key role in the synthesis of Leucinostatin, and the synthesis of leucinostatin of
P. lilacinum was suggested. This hypothetical biosynthesis is initiated by the assembly of 4-methylhex-2-enoic acid via reductive PKs. However, they were unable to estimate which PKs were responsible for 4-methylhex-2-enoic acid
[18].
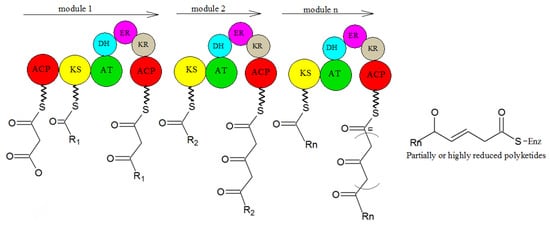
In microorganisms, PK comes from the independent hypothesis of a variety of compounds. Polyketide compounds are assembled by repeated Claisen condensations between the activated acyl initiation unit and the chain extender unit derived from malonyl-CoA. This process is catalyzed by the synergistic action of keto synthase (KS), acyltransferase (AT), and phosphopan ethylation acyl carrier protein (ACP) or CoA linked to the primary chain. After each extension step, the functionality of β-keto can be reduced by further involved enzymes
[33][76]. This general PK catalytic mechanism is realized by different enzyme mechanisms (
Figure 12). Three types of PKs are described below, which are responsible for the biosynthesis of polyketide chains.
Figure 12. The biosynthesis of PKs
[34][77].
PKs can be divided into three types: type I PKs are modular enzymes composed of several functional domains, which are arranged linearly and covalently. Any functional domain is not reused in the process of chain synthesis and extension. They mainly synthesize polyether, polyene, and macrolides. PKs of type II are aromatic, starting from acetyl CoA. Polyketones with an aromatic ring structure are synthesized with malonyl coenzyme A as an extension unit. Type III PKs are chalcone synthetases, a kind of homologous dimer enzyme that can be reused. It catalyzes the condensation of acetyl-CoA molecules to synthesize one ring or multi-ring aromatic polyketones
[31][74].
It is generally believed that most α-pyrones are synthesized through the polyketide pathway
[35][78]. Terpenoids are a kind of chain or cyclic secondary metabolites, which are composed of isoprene as the basic unit. Terpenoids are synthesized by terpene synthase and can be divided into: monoterpenes, with geranyl diphosphate as the synthetic precursor; sesquiterpenes, with farnesyl diphosphate as the synthetic precursor; diterpenes, with geranyl pyrophosphate as the precursor. According to the degree of reduction, it can be divided into reduced terpenoids and nonreduced terpenoids
[36][79].
3. Problems and Perspectives
Among the more than 40 metabolites reviewed in this paper, we can see that most SMs of
P. lilacinus that have been reported so far have the functions of anticancer activity, antimicrobial activity, insecticidal activity, cytotoxicity, drug carriers, and so on. Most importantly, some of the compounds showed potent activities compared to those of the positive controls, which indicates that they could be used to develop new medicines. These include the anticancer lead compound leucinostatins, ergosterol peroxide, (22E,24R)-5α, 6α-epoxy-3β-hydroxyergosta-22-ene-7-one, and paecilaminol. Leucinostatins is cytotoxic to HeLa cells, Ehrlich subcutaneous solid tumors, and prostate cancer. However, it was found to be toxic to rats by intraperitoneal injection, so more attention should be paid to its safety assessment when developing the drug. The other three compounds have the ability to inhibit human cancer K562, MCF-7, HL-60, and BGC-823 cells, but their safety for other species is still unknown. Acremoxanthone and acremonidin were both calmodulin inhibitors; paecilomide is an acetylcholinesterase inhibitor and kojic acid showed tyrosinase inhibitory activity, indicating their potential as insecticides. These remarkable activities make many of these compounds suitable candidates for new drugs and insecticides discovery and may lead to future synthesis studies. However, some of the SMs of
P. lilacinus are toxic to animals and humanity. Hocquette, Dr. Qian, Pastor, and others have reported infections caused by
P. lilacinus in immunocompromised patients
[37][38][80,81].
With the development of society, more and more attention has been paid to biological control, more and more fungal products will come out, and the safety of related products has also received great attention. Therefore, how to ensure the safety of fungal products has become particularly important.
Generally, in production and in life, there are six destinations (i.e., target organisms, nontarget organisms, soil, water, atmosphere, and humans) involved in the production and application of
P. lilacinum pesticide formulations. The most important destination is target organisms, including pests and crops when preparations are released in fields. Soil is another important destination, especially when it is released through soil treatments for nematodes. Water and the atmosphere are the destinations of the drifting formulations. Humans contact
P. lilacinum through direct and indirect pathways. There is no doubt that the biosafety risks of
P. lilacinum are closely related to the sources and fates of the SMs produced by entomopathogenic fungi
[39][24].
Surveying the SMs will be beneficial to improving the safety of P. lilacinum fungal products. Thus, developing the discovery, structure, function, and synthesis pathway of secondary metabolites of P. lilacinum are of great significance to biomedicine, human health, and agricultural disease control. For a long time, due to the gene silencing or low expression of most gene clusters in common culture medium, the research of fungal secondary metabolites has been hindered to some extent. There are only a few kinds of research on SMs of P. lilacinum, which are leucinostatins, acremoxanthones, and paecilomides, and their synthetic pathway and regulatory mechanism are still unclear. Therefore, it is necessary to use a super-expressing transcription factor, to replace the promoter in the synthetic gene cluster with an inducible strong promoter, to modify the histone, to heterologously express the gene cluster to activate the silent gene cluster, and to further discover that the structure is novel and biologically active. The SMs production yield of P. lilacinum needs to be improved by changing the culture conditions. First, gene knockout methods need to be used to further clarify the synthesis mechanism of secondary metabolites. In addition, it is necessary to continuously improve the efficiency and precision of chemical separation detection, in order to be more conducive to the separation of secondary metabolites and the identification of structural functions.