Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Oscar Molina and Version 2 by Yvaine Wei.
B-cell acute lymphoblastic leukemia (B-ALL) is characterized by an uncontrolled proliferation of blood cells in the bone marrow. Hypodiploidy with less than 40 chromosomes is a rare genetic abnormality in B-cell acute lymphoblastic leukemia (B-ALL). This condition can be classified based on modal chromosome number as low-hypodiploidy (30–39 chromosomes) and near-haploidy (24–29 chromosomes), with unique cytogenetic and mutational landscapes.
- hypodiploidy
- near-haploidy
- B-cell acute lymphoblastic leukemia
1. Introduction
Acute lymphoblastic leukemia (ALL) is a neoplasm arising from lymphoid precursor cells and can be classified as B-ALL or T-ALL based on the immunophenotype of the neoplastic cells [1]. The global incidence of ALL is ~3 cases per 100,000 people and shows a bimodal distribution, with a predominant peak early in life (1 to 15 years) and a second, much lower, peak in older groups (>55 years) [2] (Figure 1). ALL has a slightly higher incidence in males, with a male-to-female ratio of 1.2:1 [3]. The disease is characterized by the uncontrolled proliferation of leukemic cells, which invade the bone marrow (BM), peripheral blood (PB), and other hematopoietic tissues including spleen, liver, and lymph nodes, resulting in a hematopoietic displacement which is responsible for the cytopenias frequently observed at diagnosis. ALL cells also infiltrate commonly the central nervous system (CNS).
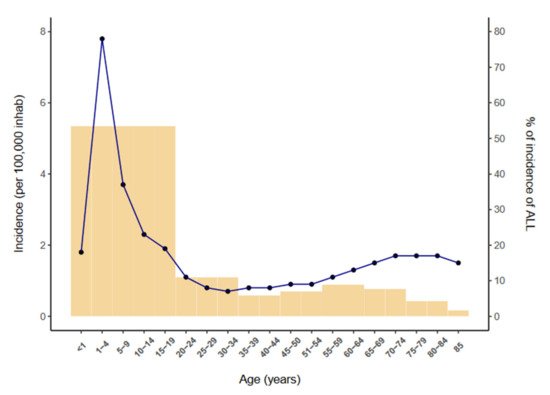
Figure 1.
B-cell precursor ALL (B-ALL) accounts for 80–85% of ALL cases and is characterized by small-medium sized leukemic blast cells staining almost always positive for the B-cell antigens CD19, cytoplasmic CD79a and CD22. Although BM and PB are involved in most cases, B-ALL occasionally presents with primary nodal or extranodal sites (B-lymphoblastic lymphoma), which predominantly affect skin, soft tissue, bone and lymph nodes [4].
2. Definition of Hypodiploid B-ALL Subgroups
Hypodiploidy -the loss of one or more whole chromosomes- is a rare cytogenetic finding (≤7%) in children and adults with B-ALL and is generally an adverse prognostic marker [5][6][7][8][9][10][11][12][13][14][15][16][12,16,17,18,19,20,21,22,23,24,25,26]. Most cases (~80%) of hypodiploid B-ALL present with 45 chromosomes and are classified as near-diploid B-ALL, a clinically distinct entity characterized by rearrangements that form dicentric chromosomes but that does not have outcomes as poor as those associated with hypodiploid B-ALL [11][21].
3. Cytogenetic Characterization of B-ALL with Hypodiploidy <40 Chromosomes
Hypodiploid cases with <40 chromosomes can be further subdivided into two groups based on the bimodal distribution of chromosome numbers: (i) near-haploidy, with 24–29 chromosomes (Figure 2A), and (ii) low-hypodiploidy, with 30–39 chromosomes (Figure 2B,C) [17][27] (Table 13). Although the modal number of chromosomes is variable, the most recurrent modal numbers are 25–28 for near-haploid and 33–39 for low-hypodiploid B-ALL [18][36]. Based on conventional chromosome banding analyses, hypodiploidy with <40 chromosomes shows a non-random loss of chromosomes: in near-haploid B-ALL, retained disomies generally comprise chromosomes 8, 10, 14, 18, 21, X and Y [11][12][19][20][21,22,30,37] whereas in low-hypodiploid cases, retained disomies are more variable and typically comprise chromosomes 1, 5, 6, 8, 10, 11, 14, 18, 19, 21, 22, X and Y, with retained disomies for chromosomes 1, 6, 11 and 18 being the most frequently observed. The most typically lost chromosomes are chromosome 3, 7, 9, 15, 16 and 17 [11][12][19][21][22][21,22,30,38,39]. The non-random retention of chromosomes suggests that these chromosomes may harbor specific genes that enhance the oncogenic potential of leukemic cells.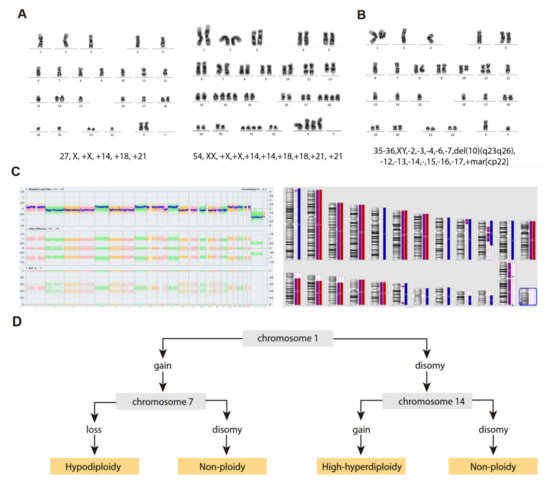
Figure 2. Cytogenetic characterization of B-ALL with <40 chromosomes. (A) G-banded karyotype of near-haploid B-ALL leukemic cells. Left panel, near-haploid clone. Right panel, chromosomally-doubled clone of the same patient. (B) G-banded karyotype of low-hypodiploid B-ALL leukemic cells. Karyotype formulas are indicated below. (C) SNP-array karyogram obtained for the low-hypodiploid B-ALL patient in B. Right panel, blue bars indicate chromosomal disomies of the duplicated/near-triploid clone, red bars indicate chromosomal losses, and purple bars indicate absence of heterozygosity. Left panel, Log2 ratio plot detailing whole chromosomal view for each chromosome, the figure demonstrates pattern of low-hypodiploidy where chromosomes with the lowest Log2 ratio represent the monosomies and a partial deletion of chromosome 10. Allele difference plot and B-allele frequency plot (BAF; BB, AB and AA alleles) indicates copy-neutral loss of heterozygosity. (D) Algorithm proposed by Creasey et al. [22][39] to distinguish hypodiploid with <40 chromosomes and high-hyperdiploid B-ALL cases based on specific chromosomal gains.
Table 1. Cytogenetic characteristics of B-ALL patients with <40 chromosomes.
“Masked Hypodiploidy”: A Clinical Challenge
| Age (Years) | Near-Haploid | Low-Hypodiploid | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MN | Retained chr | Lost chr | Doubled Clone | Frequency | MN | Retained chr | Lost chr | Doubled Clone | Frequency | ||
| 1–18 | 25–28 | 8, 10, 14, 18, 21 and sex chr. | yes | 0.0046 | 30–40 | 1, 19, 21, 22 and sex chr. | 3, 7, 13, 16, 17 | yes | 0.41 | [7] | |
| 1–10 | 24–28 | 8, 10, 14, 18, 21 and sex chr. | 7, 13, 14, 20, X | yes | 0.0042 | 33–44 | 7, 13, 14, 20, X | nr | 0.79 | [9] | |
| 2–15 | 23–29 | 14, 18, 21 and sex chr. | yes | 0.0039 | 33–39 | 1, 2, 5, 6, 8, 10, 11,12, 14, 18, 19, 21, 22 and the sex chr. | 7, 17 | yes | 0.39 | [11] | |
| 15–84 | - | - | - | - | - | 30–39 | 1, 5, 6, 8, 10, 11, 15, 18, 19, 21, 22, X, Y | 3, 7, 15, 16, 17 | 66 to 78 chr | 0.05 | [23] |
| 15–55 | - | - | - | - | - | 33–39 | nr | nr | nr | 0.0008 | [11] |
| 15–55/>55 | <30 | 0.0016 | 32–39 | 1 | 2, 3, 7, 9, 13, 15, 16, 17, 20, 4 | 64–74 | 3.85% | [24] | |||
| 1–9/>10 | 24–29 | 14, 18, 21 and sex chr. | nr | nr | nr | 33–39 | nrec | 3, 7, 16, 17 | nr | nr | [25] |
| <31 | 24–31 | 14, 18, 21 and sex chr. | nr | yes | 0.008 | 32–39 | 1, 8, 10, 11, 18, 19, 21 and 22 | nr | nr | 0.0064 | [26] |
Abbreviations: chr, chromosomes; MN, modal numbers; nr, non-reported; nrec, non-recurrent.
“Masked Hypodiploidy”: A Clinical Challenge
A feature common to patients with near-haploid and low-hypodiploid B-ALL is the presence of a clone with an exact or near-exact chromosome doubling of the hypodiploid clone, resulting in a clone with a modal chromosome number of 50–78, in the high-hyperdiploid or triploid range [7][11][12][26][17,21,22,41] (Table 13) (Figure 2A). Notably, some chromosomes are preferentially lost after chromosome doubling; typically, chromosomes 2, 5, 6, 10, 14 and 22 [21][38]. The presence of hypodiploid doubled clones has been observed in ~60–65% of patients with near-haploid and low-hypodiploid B-ALL in different studies, and is commonly observed as a mosaic with both hypodiploid and hyperdiploid (doubled) clones visible by standard cytogenetics, fluorescence in situ hybridization (FISH) or flow cytometry analysis of DNA content [11][26][27][21,41,43]. Furthermore, the doubled clone may be the only one detected at diagnosis, leading to the manifestation known as “masked hypodiploidy”, which is clinically challenging since patients can be erroneously classified and treated for high-hyperdiploid B-ALL while being at higher risk of treatment failure. It has been reported that there is no difference in clinical outcome for patients with “masked hypodiploidy”, those who are mosaic for a doubled clone and a hypodiploid clone, and those who have only a hypodiploid clone [12][28][26][22,35,41]. In addition, the hypodiploid clone tends to be quantitatively more frequent at relapse, suggesting that the actual hypodiploid clones may be more chemoresistant than their hyperdiploid (doubled) counterparts [10][29][20,44].
4. Molecular Characterization of Hypodiploid B-ALL with <40 Chromosomes
In addition to the massive genetic losses, both near-haploid and low-hypodiploid B-ALL show characteristic and differentiated gene expression profiles, in addition to specific mutational and focal copy-number alteration (CNA) landscapes, those excluding whole chromosome losses [19][30]. Notably, near-haploid and low-hypodiploid B-ALL presenting with or without doubled clones show similar transcriptional and mutational profiles [19][30], most likely explaining the similar clinical outcomes between patients with and without chromosomal doubling [7][12][26][17,22,41].4.1. Near-Haploid B-ALL
The mutational landscape of near-haploid B-ALL is characterized mainly by the presence of alterations involving receptor tyrosine kinases and activating RAS signaling alterations, with >70% of patients showing mutations or focal CNA involving genes in these pathways (Table 24) [19][21][30][30,38,51]. The different RAS signaling alterations have been shown to be mutually exclusive, suggesting that, in contrast to the convergent evolution for RAS mutations observed in infants with MLL-rearranged B-ALL [31][52], a single alteration in the pathway is sufficient to maintain constitutive RAS-pathway activation. Focal deletions or point mutations in NF1 gene are the most recurrent genetic alterations of near-haploid B-ALL (≥44% of patients) [13][19][32][23,30,53].
Table 2. Molecular characteristics of hypodiploid <40 chromosomes B-ALL (Adapted from Holmfelt et al., 2013 [19]).
4.2. Low-Hypodiploid B-ALL
| Genes | Cellular Pathway | Near-Haploid B-ALL | Low-Hypodiploid B-ALL | ||||
|---|---|---|---|---|---|---|---|
| Mutation | Focal Deletion | Focal DEL + Mut | Mutation | Focal Deletion | Focal DEL + Mut | ||
| NF1 | RTK/RAS pathway | 11/68 (16%) | 16/68 (24%) | 3/68 (4%) | 0 | 2/34 (6%) | 0 |
| KRAS | 2/68 (3%) | 0 | 0 | 0 | 0 | 0 | |
| NRAS | 10/68 (15%) | 0 | 0 | 0 | 0 | 0 | |
| PTPN11 | 1/68 (1%) | 0 | 0 | 0 | 0 | 0 | |
| FLT3 | 6/68 (9%) | 0 | 0 | 0 | 0 | 0 | |
| CRLF2 | 0 | 2/68 (3%) * | 0 | 0 | 0 | 0 | |
| MAPK1 | 1/68 (1%) | 0 | 0 | 0 | 0 | 0 | |
| GAB2 | 0 | 2/68 (3%) | 0 | 0 | 1/34 (3%) | 0 | |
| EPHA7 | 0 | 2/68 (3%) | 0 | 0 | 0 | 0 | |
| RASA2 | 0 | 2/68 (3%) | 0 | 0 | 0 | 0 | |
| IKZF1 | B-cell development | 0 | 3/68 (4%) | 0 | 0 | 1/34 (3%) | 0 |
| IKZF2 | 1/68 (1%) | 0 | 0 | 0 | 18/34 (53%) | 0 | |
| IKZF3 | 1/68 (1%) | 8/68 (12%) | 0 | 0 | 1/34 (3%) | 0 | |
| PAX5 | 1/68 (1%) | 4/68 (6%) | 0 | 0 | 2/34 (6%) | 0 | |
| EBF1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| VPREB1 | 0 | 3/68 (4%) | 0 | 0 | 2/34 (6%) | 0 | |
| CDKN2A/B | Cell cycle and apoptosis | 0 | 15/68 (22%) | 0 | 0 | 8/34 (24%) | 0 |
| TP53 | 2/68 (3%) | 0 | 0 | 31/34 (91%) | 0 | 0 | |
| RB1 | 2/68 (3%) | 3/68 (4%) | 1/68 (1%) | 5/34 (15%) | 8/34 (24%) | 0 | |
| ETV6 | Hematopoiesis | 1/68 (1%) | 3/68 (4%) | 1/68 (1%) | 0 | 0 | 0 |
| Histone cluster (6p22) | Histone-related | 0 | 13/68 (19%) | 0 | 0 | 1/34 (3%) | 0 |
| ARID1B | 0 | 2/68 (3%) | 0 | 0 | 0 | 0 | |
| PAG1 | BCR signalling | 1/68 (1%) | 6/68 (9%) | 0 | 0 | 1/34 (3%) | 0 |
| ARPP21 | Calmodulin signalling | 0 | 1/68 (1%) | 0 | 0 | 0 | 0 |
| SLX4IP (C20orf194) | Telomere length maintenance | 0 | 2/68 (3%) | 0 | 0 | 0 | 0 |
| CUL5 | Ubiquitin pathway | 0 | 2/68 (3%) | 0 | 0 | 0 | 0 |
| FAM53B | Wnt signalling | 0 | 2/68 (3%) | 0 | 0 | 0 | 0 |
| PDS5B (APRIN) | Cohesin complex | 0 | 2/68 (3%) | 0 | 0 | 0 | 0 |
| ANKRD11 | Cell adhesion | 0 | 0 | 0 | 0 | 2/34 (6%) | 0 |
| DMD | 0 | 0 | 0 | 0 | 1/34 (3%) | 0 | |
* one patient encoding P2RY8-CRLF2.
4.2. Low-Hypodiploid B-ALL
The genetic hallmark of low-hypodiploid B-ALL is TP53 mutations, which are observed in >90% of patients in both childhood and adult low-hypodiploid B-ALL [19][21][30][33][30,38,51,55]. Most are missense mutations in exons 5–8, affecting the DNA-binding domain and the nuclear localization sequence [19][21][30,38]. Other characteristic and recurrent genetic alterations in the low-hypodiploid B-ALL subtype are RB1 mutations or deletions (41% of cases), deletions of IKZF2/Helios (53% of cases) and deletions of CDKN2A/B genes (22% of cases) [19][21][30,38]. Mutations of TP53 are found in homozygosity in virtually all low-hypodiploid B-ALL cases due to the very recurrent loss of chromosome 17. TP53 mutations are frequently found in non-tumor hematopoietic cells in 50% of the cases of childhood low-hypodiploid B-ALL [21][30][38,51], suggesting that these cases may be a manifestation of Li-Fraumeni syndrome or other germline TP53 cancer-predisposing mutations [19][33][34][30,55,56]. Accordingly, genetic counseling is recommended for children with low-hypodiploid B-ALL carrying TP53 mutations, and their relatives [35][36][57,58]. In contrast to childhood cases, TP53 mutations in low-hypodiploid adult B-ALL are somatic, are not found in healthy hematopoietic cells, and not detectable in remission samples [19][21][30,38].5. Etiology of Hypodiploidy in B-ALL
Genomic analyses of these subtypes have been difficult given the limited number of cases; however, a study on a small cohort of 8 near-haploid and 4 low-hypodiploid B-ALL samples suggested that the massive loss of chromosomes is the primary oncogenic event, with other oncogenic insults occurring after hypodiploidy [20][37]. This is consistent with similar analyses in high-hyperdiploid B-ALL cases, the most frequent aneuploid entity in B-ALL, indicating that chromosome gains were the primary oncogenic event [37][38][60,61]. Thus, similar pathogenic mechanisms involving gross aneuploidies may be shared in these B-ALL subtypes. Furthermore, the genomic landscape of near-haploid and low-hypodiploid B-ALL subtypes, as well as that of high-hyperdiploid subtypes, is characterized by aneuploidy and subtype-specific mutations, with significant fewer microdeletions and structural chromosomal rearrangements in comparison with other cytogenetic subtypes containing structural chromosomal reorganizations [19][37][30,60]. Collectively, these data strongly suggest that hypodiploidy has a direct impact on cell transformation and leukemogenesis rather than being solely a passenger event. The fact that severe hypodiploidy is observed in a wide spectrum of neoplasms further indicates that it is indeed a major contributor of tumorigenesis [39][62].6. Outcome and Treatment Strategies for B-ALL with Hypodiploidies <40 Chromosomes
6.1. Relationship of Genetic and Clinical Features with Patient Outcome
The EFS is not significantly different between patients with near-haploid or low-hypodiploid B-ALL, including those cases with “masked hypodiploidy” [13][14][35][23,24,57]. In some cases, hypodiploidy may accompany other primary genetic abnormalities, such as BCR-ABL1, TCF3-PBX1, ETV6-RUNX1 and KMT2A rearrangements, which modulate the prognosis of the disease. Accordingly, some authors have suggested that these patients should be treated based on the primary structural abnormalities rather than the hypodiploidy, and on their MRD values after induction [14][24]. The high presence of germline TP53 mutations among patients with low-hypodiploidy confer an increased risk of relapse in this group and is associated with the development of secondary neoplasms [14][24]. Therefore, it is highly recommended that all patients with low-hypodiploidy B-ALL are tested for germline TP53 mutations [14][40][24,69]. Strikingly, the germline TP53 mutations in these cases have been associated with increased mortality due to second neoplastic malignancies following hematopoietic stem cell transplantation (HSCT), highlighting the importance of the germline study in low-hypodiploid B-ALL to assess HSCT versus less toxic alternative therapies [16][41][42][26,67,70].
