Arginine availability and activation of arginine-related pathways at cancer sites have profound effects on the tumor microenvironment, far beyond their well-known role in the hepatic urea cycle. Arginine metabolism impacts not only malignant cells but also the surrounding immune cells behavior, modulating growth, survival, and immunosurveillance mechanisms, either through an arginase-mediated effect on polyamines and proline synthesis, or by the arginine/nitric oxide pathway in tumor cells, antitumor T-cells, myeloid-derived suppressor cells, and macrophages.
- arginine
- arginase
- metabolism
- tumor microenvironment
1. Arginine Metabolism
Arginine, the substrate of arginase, is a nonessential cationic amino acid obtained from diet, endogenous synthesis, and protein turnover and is essential for protein synthesis and a precursor of several molecules such as urea, nitric oxide, polyamines, proline, and agmatine, among others [14][1] (Figure 1).
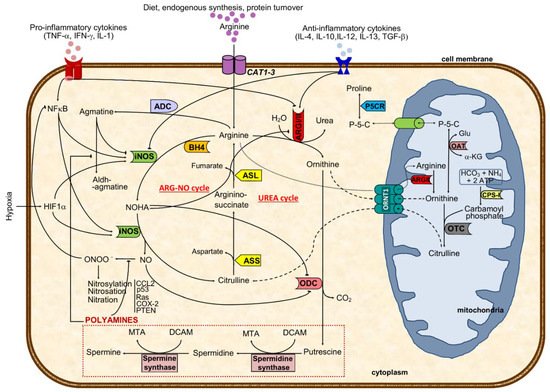
2. Arginine Metabolism and Cancer
High arginase activity has been described in different malignancies, including of the skin, colon, breast, hematologic, and prostate, mainly due to their need to produce polyamines and respond to rapid proliferation [50,51,52,53,54,55][9][10][11][12][13][14]. In agreement, tumor cell lines (some resistant to ODC inhibitors) holding arginase activity resulted in lower production of polyamines, reduced cell proliferation, and induction of apoptosis after inhibition of ARG activity [56,57][15][16]. Besides the mechanism of substrate deprivation for cancer growth, in which arginases and arginine availability have a nearly direct effect in cancer cells, current perspectives elicited an extended explanation focused on the interplay of arginases metabolism and arginine among components of the tumor microenvironment as mediators of immunosurveillance. Notably, the efficacy of chimeric antigen receptor T cells (CAR-T) in hematological and solid malignancies has been shown to be compromised by depletion of arginine due to the low expression of arginine resynthesis enzymes, ASS, and ornithine transcarbamylase; therefore, the re-engineering of T cells may correct the mentioned defect and induce the expression of functional ASS or ornithine transcarbamylase enzymes, increasing CAR-T cell proliferation without compromising function [58][17]. Arginine depletion exerted target transcriptional and posttranscriptional regulation of ASS and ASL activities and regulation of other genes such as those coding for iNOS, CAT-1 or T-cell receptor (TCR) zeta-chain, thereby modulating relevant cellular mechanisms (Figure 2): (i) there is a diminished efficiency in iNOS mRNA translation and protein stability triggered by lower extracellular arginine and arginases overactivity due to the downregulation of eukaryotic initiation factor 2α (eIF2α) [18,23,42][5][18][19]; (ii) the lower TCR zeta-chain mRNA stability and expression affects lymphocytes proliferative capability [29,59][20][21]; (iii) both transcriptional and translational efficiency of CAT-1 mRNA are increased [24[22][20],29], conferring advantage for capturing extracellular arginine to tumor cells. During tumor progression, macrophage polarization switches from classically activated M1, which promotes tumor initiation and adaptive immunity activation towards an alternatively activated (M2) polarized state, favoring tumor progression and spread [60][23]. In M1, inflammatory mediators regulate ASS expression together with iNOS, ASL, and CAT-2 activities, thus providing increased arginine synthesis and uptake for NO production. This regulation of arginine and NO by inflammatory mediators is also found in other cell types (e.g., epithelial cells and vascular smooth muscle) and establishes the arginine-NO pathway that supports NO generation (Figure 1) [19,24][6][22]. M1 macrophages may contribute to the eradication of cancer cells once they generate NO, pro-inflammatory cytokines, and chemokines, or may act as antigen-presenting cells to activate CD8+ cytotoxic T-cells [61][24]. Conversely, M2 macrophages promote tumorigenesis through anti-inflammatory cytokines and chemokines (IL-4, IL-13, and transforming growth factor β, TGF-β pathways) [61][24] (Figure 2). In macrophages, both ARGI and ARGII and NOS expression and activity were identified [31,33][25][26]. ARGI is markedly induced in human mononuclear cells after tissue injury, associating with decreases in arginine and NO availability [33,39][26][27]. ARGI is inducible, while ARGII is constitutively expressed in macrophages and tightly regulated by Th1 and Th2 cytokines that modulate the production of ornithine and subsequent products [32,33][28][26]. Indeed, pro-inflammatory cytokines (tumor necrosis factor alpha, TNF-α; interleukin 1, IL-1; interferon gamma, IFN-γ) induce iNOS and suppress arginase activity, whereas anti-inflammatory cytokines (IL-4, IL-12, IL-13, and IL-10) reduce iNOS and increase arginase activity [18,21,32][5][8][28] (Figure 2). As in epithelial cells, also in macrophages, the synthesis of NO is dependent on arginase expression, which is well correlated with arginase activity [21,39,67][8][27][29]. The key inflammation-related transcription factors nuclear factor erythroid 2-like 2 and nuclear factor kappa-B can be modulated by NO in malignant cells promoting cell survival and tumor progression [69][30]. Even though tumor cell-induced iNOS is involved in those processes through NO production, its activation in macrophages further increases the inflammatory burden [36,65][31][32]. Additionally, arginase induction in macrophages decreases NO production by conversion of arginine in ornithine [22,36,39,46,48][33][31][27][34][35]. In this process, ARGII and ODC are initially induced and may lead to increased polyamine production [33][26]; and then followed by iNOS downregulation, further relocating arginine to ornithine synthesis that can be used for polyamines and proline synthesis, essential for cell proliferation and tissue remodeling [18,21,33,39,70][5][8][26][27][36].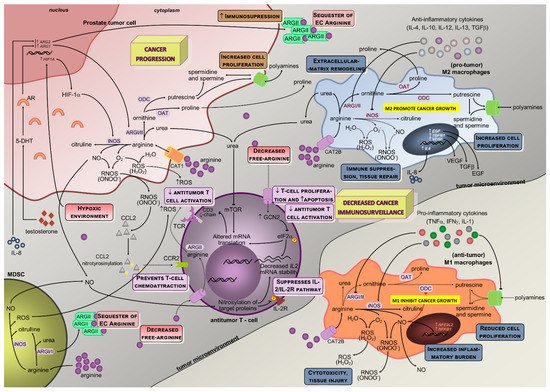
3. Modulation of Arginine Metabolism in Oncology
Modulation of arginine availability and arginase activity by targeting the enzymes in its metabolic pathway may have a role in cancer therapeutics. The auxotrophic affinity of cancer cells for specific amino acids has been the rationale for therapeutic deprivation regimens, where arginine fits, once it is required by proliferating cells, despite nonessential to normal cells. Downregulation of the rate-limiting arginine-producer enzyme ASS in tumor cells, which is common in most cancers [83][38], correlates with the dependence on extracellular arginine due to the inability to produce endogenous arginine for growth [56][15]. Thus, arginine-depleting enzymes involved in arginine catabolism out of the cell (arginase and arginine deiminase, androgen-independent, a microbial enzyme that converts arginine to citrulline and ammonia) may have an antitumor effect, with tumoral ASS deficiency serving as a prognostic biomarker and predictor of sensitivity to arginine deprivation therapy [57][16].
Citrullination is a deimination of protein-embedded arginine, which is converted to the non-coded amino acid citrulline [93][39]. This process is catalyzed by a family of enzymes called peptidyl arginine deiminases. Citrullination is involved in disease pathogenesis with the involvement of the following mechanisms epigenetic, pluripotency, immunity, and transcriptional regulation. Indeed, peptidyl arginine deiminase 2-mediated arginine citrullination might have an implication on transcriptional regulation in cancer [94][40]. Furthermore, citrullination of histones in neutrophils facilitates neutrophil extracellular trap formation or NETosis [95][41].
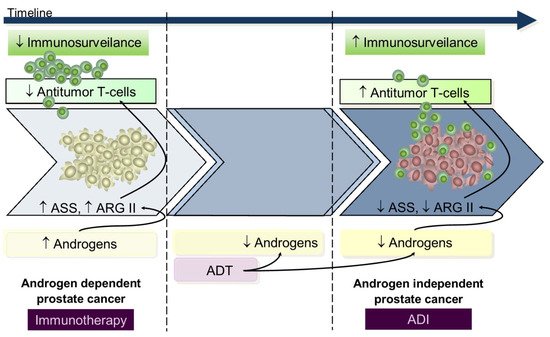
Data from in vitro and in vivo studies using either in androgen-dependent or androgen-independent prostate cancer cell lines suggests that during the androgen-dependent phase, with higher ASS and ARGII expression, tumors were resistant to androgen-independent, whereas in the androgen-independent stage, presenting decreased ASS and ARGII expression, there was a response to androgen-independent treatment [50,92][9][42]. From this perspective, we hypothesize that during the early androgen-dependent PCa development preeminent mechanisms contribute to immunosuppression rendering immunotherapy a key role; on the contrary, advanced androgen-independent tumors that survive in low androgen environments have increased T-cells infiltrated [97][43] and low ASS and ARGII expression [50,92][9][42] rendering androgen-independent a potential therapeutic utility since lymphocyte activity is already restored (Figure 3); however, despite these findings from in vitro and animal models [56[15][44][45][46],98,99,100], and in patients with metastatic cancer [101,102,103][47][48][49], the apparently low potency of ARG and AD androgen-independent immunogenicity advise restrained enthusiasm.
References
- Bhagavan, N.V.; Ha, C.-E. Chapter 15—Protein and Amino Acid Metabolism. In Essentials of Medical Biochemistry, 2nd ed.; Bhagavan, N.V., Ha, C.-E., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 227–268.
- Bahadoran, Z.; Carlström, M.; Mirmiran, P.; Ghasemi, A. Nitric oxide: To be or not to be an endocrine hormone? Acta Physiol. 2020, 229.
- Morris, S.M., Jr. Arginases and arginine deficiency syndromes. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 64–70.
- Morris, S.M., Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 2002, 22, 87–105.
- Satriano, J. Arginine pathways and the inflammatory response: Interregulation of nitric oxide and polyamines: Review article. Amino Acids 2004, 26, 321–329.
- Morris, S.M., Jr. Arginine metabolism: Boundaries of our knowledge. J. Nutr. 2007, 137, 1602S–1609S.
- Pawar, H.; Puri, M.; Fischer Weinberger, R.; Madhubala, R.; Zilberstein, D. The arginine sensing and transport binding sites are distinct in the human pathogen Leishmania. PLoS Negl. Trop. Dis. 2019, 13, e0007304.
- Grillo, M.A.; Colombatto, S. Arginine revisited: Minireview article. Amino Acids 2004, 26, 345–351.
- Gannon, P.O.; Godin-Ethier, J.; Hassler, M.; Delvoye, N.; Aversa, M.; Poisson, A.O.; Peant, B.; Alam Fahmy, M.; Saad, F.; Lapointe, R.; et al. Androgen-regulated expression of arginase 1, arginase 2 and interleukin-8 in human prostate cancer. PLoS ONE 2010, 5, e12107.
- Tabe, Y.; Lorenzi, P.L.; Konopleva, M. Amino acid metabolism in hematologic malignancies and the era of targeted therapy. Blood 2019, 134, 1014–1023.
- Singh, R.; Pervin, S.; Karimi, A.; Cederbaum, S.; Chaudhuri, G. Arginase activity in human breast cancer cell lines: N(omega)-hydroxy-L-arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res. 2000, 60, 3305–3312.
- Buga, G.M.; Wei, L.H.; Bauer, P.M.; Fukuto, J.M.; Ignarro, L.J. NG-hydroxy-L-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am. J. Physiol. 1998, 275, R1256–R1264.
- Tate, D.J., Jr.; Vonderhaar, D.J.; Caldas, Y.A.; Metoyer, T.; Patterson, J.R.T.; Aviles, D.H.; Zea, A.H. Effect of arginase II on L-arginine depletion and cell growth in murine cell lines of renal cell carcinoma. J. Hematol. Oncol. 2008, 1, 14.
- Massi, D.; Marconi, C.; Franchi, A.; Bianchini, F.; Paglierani, M.; Ketabchi, S.; Miracco, C.; Santucci, M.; Calorini, L. Arginine metabolism in tumor-associated macrophages in cutaneous malignant melanoma: Evidence from human and experimental tumors. Hum. Pathol. 2007, 38, 1516–1525.
- Feun, L.; You, M.; Wu, C.J.; Kuo, M.T.; Wangpaichitr, M.; Spector, S.; Savaraj, N. Arginine deprivation as a targeted therapy for cancer. Curr. Pharm. Des. 2008, 14, 1049–1057.
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer. J. Int. Du Cancer 2010, 126, 2762–2772.
- Fultang, L.; Booth, S.; Yogev, O.; Martins da Costa, B.; Tubb, V.; Panetti, S.; Stavrou, V.; Scarpa, U.; Jankevics, A.; Lloyd, G.; et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood 2020, 136, 1155–1160.
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336 Pt 1, 1–17.
- Morris, S.M., Jr. Arginine: Beyond protein. Am. J. Clin. Nutr. 2006, 83, 508S–512S.
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004, 64, 5839–5849.
- Morris, S.M., Jr. Recent advances in arginine metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 45–51.
- Morris, S.M., Jr. Recent advances in arginine metabolism: Roles and regulation of the arginases. Br. J. Pharmacol. 2009, 157, 922–930.
- Nath, N.; Kashfi, K. Tumor associated macrophages and ‘NO’. Biochem Pharm. 2020, 176, 113899.
- Ostrand-Rosenberg, S. Immune surveillance: A balance between protumor and antitumor immunity. Curr. Opin. Genet. Dev. 2008, 18, 11–18.
- Louis, C.A.; Reichner, J.S.; Henry, W.L., Jr.; Mastrofrancesco, B.; Gotoh, T.; Mori, M.; Albina, J.E. Distinct arginase isoforms expressed in primary and transformed macrophages: Regulation by oxygen tension. Am. J. Physiol. 1998, 274, R775–R782.
- Mori, M.; Gotoh, T. Arginine metabolic enzymes, nitric oxide and infection. J. Nutr. 2004, 134, 2820S–2825S; discussion 2853S.
- Mori, M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J. Nutr. 2007, 137, 1616S–1620S.
- Munder, M.; Eichmann, K.; Moran, J.M.; Centeno, F.; Soler, G.; Modolell, M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 1999, 163, 3771–3777.
- Ochoa, J.B.; Bernard, A.C.; O’Brien, W.E.; Griffen, M.M.; Maley, M.E.; Rockich, A.K.; Tsuei, B.J.; Boulanger, B.R.; Kearney, P.A.; Morris, S.M., Jr. Arginase I expression and activity in human mononuclear cells after injury. Ann. Surg. 2001, 233, 393–399.
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-kappaB and Their Concerted Modulation in Cancer Pathogenesis and Progression. Cancers 2010, 2, 483–497.
- Gotoh, T.; Mori, M. Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. J. Cell Biol. 1999, 144, 427–434.
- Sica, A.; Allavena, P.; Mantovani, A. Cancer related inflammation: The macrophage connection. Cancer Lett. 2008, 267, 204–215.
- Morris, S.M., Jr. Enzymes of arginine metabolism. J. Nutr. 2004, 134, 2743S–2747S; discussion 2765S–2767S.
- Luiking, Y.C.; Ten Have, G.A.; Wolfe, R.R.; Deutz, N.E. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189.
- Mori, M.; Gotoh, T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem. Biophys. Res. Commun. 2000, 275, 715–719.
- Kropf, P.; Fuentes, J.M.; Fahnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Muller, I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1000–1002.
- Kropf, P.; Baud, D.; Marshall, S.E.; Munder, M.; Mosley, A.; Fuentes, J.M.; Bangham, C.R.; Taylor, G.P.; Herath, S.; Choi, B.S.; et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur. J. Immunol. 2007, 37, 935–945.
- Dillon, B.J.; Prieto, V.G.; Curley, S.A.; Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: A method for identifying cancers sensitive to arginine deprivation. Cancer 2004, 100, 826–833.
- Sharma, P.; Lioutas, A.; Fernandez-Fuentes, N.; Quilez, J.; Carbonell-Caballero, J.; Wright, R.H.G.; Di Vona, C.; Le Dily, F.; Schüller, R.; Eick, D.; et al. Arginine Citrullination at the C-Terminal Domain Controls RNA Polymerase II Transcription. Mol. Cell 2019, 73, 84–96.e87.
- Beato, M.; Sharma, P. Peptidyl Arginine Deiminase 2 (PADI2)-Mediated Arginine Citrullination Modulates Transcription in Cancer. Int. J. Mol. Sci. 2020, 21, 1351.
- Holmes, C.L.; Shim, D.; Kernien, J.; Johnson, C.J.; Nett, J.E.; Shelef, M.A. Insight into Neutrophil Extracellular Traps through Systematic Evaluation of Citrullination and Peptidylarginine Deiminases. J. Immunol. Res. 2019, 2019, 2160192.
- Kim, R.H.; Coates, J.M.; Bowles, T.L.; McNerney, G.P.; Sutcliffe, J.; Jung, J.U.; Gandour-Edwards, R.; Chuang, F.Y.; Bold, R.J.; Kung, H.J. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009, 69, 700–708.
- Mercader, M.; Bodner, B.K.; Moser, M.T.; Kwon, P.S.; Park, E.S.; Manecke, R.G.; Ellis, T.M.; Wojcik, E.M.; Yang, D.; Flanigan, R.C.; et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14565–14570.
- Kelly, M.P.; Jungbluth, A.A.; Wu, B.W.; Bomalaski, J.; Old, L.J.; Ritter, G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br. J. Cancer 2012, 106, 324–332.
- Stelter, L.; Evans, M.J.; Jungbluth, A.A.; Longo, V.A.; Zanzonico, P.; Ritter, G.; Bomalaski, J.S.; Old, L.; Larson, S.M. Imaging of tumor vascularization using fluorescence molecular tomography to monitor arginine deiminase treatment in melanoma. Mol. Imaging 2013, 12, 67–73.
- Stelter, L.; Fuchs, S.; Jungbluth, A.A.; Ritter, G.; Longo, V.A.; Zanzonico, P.; Raschzok, N.; Sauer, I.M.; Bomalaski, J.S.; Larson, S.M. Evaluation of arginine deiminase treatment in melanoma xenografts using (18)F-FLT PET. Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging 2013, 15, 768–775.
- Yau, T.; Cheng, P.N.; Chan, P.; Chan, W.; Chen, L.; Yuen, J.; Pang, R.; Fan, S.T.; Poon, R.T. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Investig. New Drugs 2013, 31, 99–107.
- Yang, T.S.; Lu, S.N.; Chao, Y.; Sheen, I.S.; Lin, C.C.; Wang, T.E.; Chen, S.C.; Wang, J.H.; Liao, L.Y.; Thomson, J.A.; et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br. J. Cancer 2010, 103, 954–960.
- Szlosarek, P.W.; Luong, P.; Phillips, M.M.; Baccarini, M.; Stephen, E.; Szyszko, T.; Sheaff, M.T.; Avril, N. Metabolic response to pegylated arginine deiminase in mesothelioma with promoter methylation of argininosuccinate synthetase. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, e111–e113.
