Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Hamza Mechchate and Version 2 by Vivi Li.
Essential oils (EOs) are chemical products produced by odoriferous glands from a variety of plants. These essential oils have many health benefits: antiseptic, anti-inflammatory and antimicrobial activities. So due to these medicinal properties, the present study was designed to analyze essential oils of Thymus zygis L. and Thymus willdenowii Boiss. for their chemical composition and biological activities. These two thyme species were collected from the region of Ifrane, Middle Atlas of Morocco.
- Thymus zygis L.
- Thymus willdenowii Boiss
- volatile compounds
- GC-MS analysis
- bacteria
- fungi
- molds
- multi-resistant bacteria
1. Introduction
In recent years, there has been increasing interest in natural substances of plant origin with therapeutic potential. This increase has been linked to several factors, including beneficial health effects, in particular with the extracts and products derived from higher plants, which have led to the discovery, and the development of useful therapeutic agents [1][2][1,2]. These products are relatively low-toxic, inexpensive, available, and have effects against many pathologies (bacteria, fungus, viruses, parasites, etc.) that pose infection risks to the human body. Let us quote, for example, the essential oils whose actions against bacteria were realized in 1881 by Delacroix [3]. Since then, many essential oils have been recognized as efficient antimicrobial natural products. The activities of many essential oils have been studied during this time, like thyme, lemongrass, cinnamon, and others [4]. Numerous studies have approved their bioactivity in fighting bacteria, fungi, diabetes, oxidative stress, kidney problems, and many others [5][6][5,6]. Among the plants known for their therapeutic effects, we note thyme. This plant is commonly used as a spice and has considerable virtues thanks to the progressive discovery of its applications in care and beauty, as well as its uses in culinary practices. Thyme, a wild aromatic plant belonging to the Lamiaceae family, is found mainly in the Mediterranean region, Asia, Southern Europe, and North Africa [7]. Almost 100 species are identified throughout the world [8], and in Morocco, there are 21 species of thyme, 10 of which are endemic to Morocco (Thymus maroccanus, Thymus bleicherianus, Thymus atlanticus, Thymus satureioides, Thymus broussonnetii, Thymus leptobotrys, Thymus pallidus subsp. pallidus, Thymus pallidus subsp. eriodontus, Thymus riatarum, Thymus serpyllum) [9]. The whole plant (thym) is widely used in traditional medicine [10][11][10,11]. Its essential oil is widely used in alternative medicine as antiseptic, antispasmodic, antimicrobial, and antioxidant [12][13][14][12,13,14]. Thyme has been used in traditional Moroccan medicine in the treatment of diarrhea, fever, cough, infested zones, and wounds. It was also used as a tonic and stimulant [6][15][6,15] and has anti-inflammatory properties after topical application or oral administration [16]. The flowering tops of thyme mainly contain flavonoids (derivatives of apigenol and luteolol), phenol acids (especially caffeic and rosmarinic acids), tannins, resin, and its essential oil is very rich in terpenes, which are responsible for the majority of the pharmacological effects [17].
Considering the popular use of the plants from this family in traditional medicine to relieve certain pains and treat certain diseases [6] we have selected two species from the Middle Atlas of Morocco (Region of Ifrane), namely Thymus zygis L. and Thymus willdenowii Boiss. (commonly called Zaâitra or Azoukeni in Berber) in order to investigate and compare the chemical compositions, antioxidant, and antimicrobial activity against multidrug-resistant bacteria (Escherichia coli, Staphylococcus aureus, Acinetobacter baumannii, Shigella dysenteriae, Salmonella Typhi, and Enterobacter cloacae) fungi (yeasts (Candida albicans, Candida glabrata, and Candida spp.) and molds (Aspergillus fischeri, and Fusarium solani)) and determine their antioxidant activities using the DPPH and FRAP methods.
2. Phytochemical Study
The Yield of Essential Oils
The results of the essential oils yields obtained by hydrodistillation from samples of T. zygis and T. willdenowii are given in Table 1. With 5.25%, the T. zygis sample provided the highest yield against only 3.00% obtained with T. willdenowii. The latter remains higher compared to that obtained by El Idrissi and Idrissi (0.28%) [18] and also when compared to other thyme species in Morocco such as Thymus bleicherianus collected in Meknes (Center of Morocco) (1.71%), Thymus capitatus collected in Tetouan (North of Morocco) (1.43%) and Thymus satureioides collected in Agadir (southwest of Morocco) (0.69%) [19].
Table 1. The Eos yields of the two selected thyme species.
| Harvest Site | EO | Yield (%) |
|---|---|---|
| Azrou | T. zygis | 5.25 ± 0.01 |
| Ifrane | T. willdenowii | 3.00 ± 0.02 |
The obtained T. zygis EO yield is much higher than that harvested in Portugal by Moldão-Martins with 1.2% [20] and 3% yielded by Sotomayor et al. for T. zygis ssp. gracilis [21][22][21,22]. A similar yield (2.3 to 3.6%) was found by Jordan et al. [23]. These differences in yields of essential oils could be explained by several factors, including crop origin, genetic factors, geographical position, soil type, climatic conditions, weather, and extraction apparatus [24][25][26][24,25,26].
3. Physicochemical Characteristics of the Selected Thyme Essential Oils (Density, Refractive Index, and Brix Degree)
The density, refractive index and Brix degree are qualitative identification characteristics that may be used to evaluate the purity of essential oils. Each substance has its specific refractive index. The purity of a product is determined by how near its refractive index is to the anticipated value. According to Table 2 given below, the density of the essential oils of T. zygis (0.92 ± 0.05) was slightly higher than that of the essential oil of T. willdenowii (0.91 ± 0.05). The same observation was made on the refractive index and the Brix degree. T. zygis had the highest values with 1.50 ± 0.05 and 85.44 ± 0.05%, respectively. The density and refractive index values of T. zygis and T. willdenowii essential oils extracted by hydrodistillation are comparable to those of standards, indicating that our extracts are of excellent purity. In fact, in accordance with ISO 14715: 2010, the density of essential oils of thymol thyme (Thymus zygis (Loefl.) L.) varies between 0.91 and 0.93, and the refractive index varies between 1.494 and 1.50.
Table 2. Refractive index, degree Brix, and essential oils density of T. zygis and T. willdenowii.
| T. zygis | T. willdenowii | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Density | 0.92 ± 0.05 | 0.91 ± 0.05 | ||||||||
| 1932 | ||||||||||
| C | ||||||||||
| 18 | ||||||||||
| H | ||||||||||
| 36 | ||||||||||
| O | ||||||||||
| 0.62 | ||||||||||
| - | ||||||||||
| 46 | ||||||||||
| Cembrene C | ||||||||||
| 1940 | ||||||||||
| C | ||||||||||
| 20 | ||||||||||
| H | ||||||||||
| 18 | ||||||||||
| 0.69 | ||||||||||
| - | ||||||||||
| 47 | ||||||||||
| Hexadecanoic acid | ||||||||||
| 1960 | ||||||||||
| C | ||||||||||
| 16 | ||||||||||
| H | ||||||||||
| 32 | ||||||||||
| O | ||||||||||
| 2 | ||||||||||
| 2.31 | ||||||||||
| - | ||||||||||
| 48 | Cis | -Totarol, methyl ether | 2208 | C | 21 | H | 32 | O | - | 0.07 |
| Oxygenated monoterpenes | 32.81 | 68.7 | ||||||||
| Hydrocarbon monoterpenes | 8.95 | 27.55 | ||||||||
| Hydrocarboned sesquiterpene | 31.61 | 1.4 | ||||||||
| Oxygenated sesquiterpene | 13.35 | 1.85 | ||||||||
| Lignar esters | 8.35 | - | ||||||||
| Others | 3.62 | 0.27 | ||||||||
| Total | 98.69 | 99.84 | ||||||||
The EO of T. zygis seems to be characterized by a very interesting chemical composition, with chemical compounds in common (α-pinene, limonene, camphor, and carvacrol) and other different ones like germacrene D, which is present only in T. willdenowii. The latter composition is composed of 33.64% of alcohol, 40.58% of hydrocarbons, 7.82% of epoxides, 6.61% of ketones, 9.02% of esters and 2.32% of acids (Figure 2). This great variety and variability in the chemical composition of thyme essential oils is related to Moroccan geological and ecological diversity [35][36][35,36] which can even specify them from those from other regions all over the planet [37][38][37,38]. The presence or absence of a chemical component at any stage of development is solely controlled by the plant’s genetic history, but its concentration is influenced by both genetics and environmental variables [39].
Essential oils are likely to include thousands of molecules. A single essential oil may contain dozens, if not hundreds of distinct chemical components in wildly varied quantities. Some essential oils, on the other hand, may possess a nearly pure molecule such as Wintergreen (Gaultheria procumbens L.) which contains up to 99 percent methyl salicylate [40]. Many studies have made it possible to study the factors causing the chemical composition of thyme essential oil to vary, such as (i) harvest period (Several studies showed that the concentration of phenols (thymol and carvacrol) varies inversely with that of their precursors (p-cymene and γ-terpinene). Phenols are at their maximum level during the flowering period (June in the Northern hemisphere) and they are at their minimum during November / December period [41][42][41,42]. A study of the 1,8-cineole chemotypes in Spain found a peak level of 1,8-cineole during the growth phase of the plant [43]. The essential oil yield reaches its maximum during the period of full flowering.) (ii) Quality of the soil (which can affect the yield and quality of the essential oil) as the yield is better on calcareous soil than on sandy soil. Thymol production will be greater when the plant grows in sandy soil and less in clay soil. Limestone gives an intermediate proportion of thymol [44].)
In summary, many factors can influence the chemical composition of thyme essential oil. Regardless of the chemotype considered, the yield will be maximum at the time of flowering since physiological factors such as the stage of development of the plant and the nature of the secretory structures determine the quantity and quality of the essential oil produced [45]. Essential oils are generally more abundant in young organs. Many works have also shown the existence of a correlation between the qualitative composition of the essential oil and geographical variation [46][47][46,47]. Plant stress phenomena such as drought frequently alter the hormonal balance of the plant and modify the activity of many enzymes, as well as the expression of the genome [48].
5. Antioxidant Activity of the Essential Oils of the Two Thymes
The antiradical activity of essential oils was measured using spectrophotometry at 517 nm with the DPPH radical following the reduction of this radical accompanied by a change of color from violet to yellow. The results obtained made it possible to plot the curve of the inhibition percentage (%) as a function of the concentrations of essential oils (Figure 3A). These results show that the percentage of the free radical inhibition increased with the increase in the concentration of the EO, whether it is vitamin C (used as a positive control) or the essential oils of both thyme species. For all the concentrations tested, the inhibition percentage of vitamin C was greater than that of the two essential oils according to the IC50 values (Figure 3B). The value of IC50 is inversely related to the antioxidant capacity of a compound. It expresses the number of antioxidants necessary to decrease the concentration of the free radical by 50%. This concentration is determined graphically and expressed in μg/mL. T. zygis showed the greatest capacity for trapping DPPH, with an IC50 of the order of 6.13 ± 0.11 μg/mL against 6.78 ± 0.30 µg/mL noted for T. willdenowii. The antioxidant activity of the essential oils of T. zygis and T. willdenowii were greater than that obtained by Amarti et al. in their study concerning four essential oils of Moroccan thyme (T. capitatus, T. ciliatus, T. bleicherianus, and T. algeriensis), with an IC50 equal to 69.04 μL/mL, 74.025 μL/ mL, 77.24 μL/mL, 745 μL/mL, respectively [49].
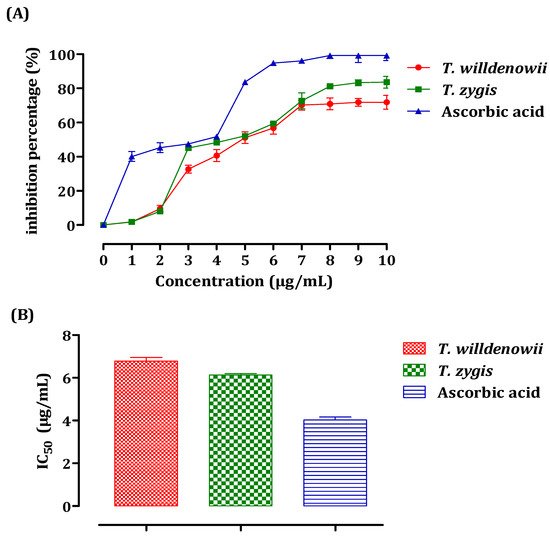

Figure 3. DPPH Free radical scavenging activity of T. zygis, T. willdenowii, and ascorbic acid. Inhibitory percentage (A) and IC50 (B) against the DPPH Free radical. data are presented as mean ± SD, the experiment was performed in a minimum of 2 replicates T. zygis: Thymus zygis, T. willdenowii: Thymus willdenowii.
Figure 4 shows the results of the evaluation of the antioxidant activity of essential oils of T. zygis and T. willdenowii by the iron reduction method (FRAP), characterized by the reduction of ferric iron Fe3+ (yellow) to ferrous iron Fe2+ (blue-green). T. zygis showed greater iron-reducing activity than that of T. willdenowii but less than that of ascorbic acid, which gave a greater iron reduction. The antioxidant activity of the two essential oils is expressed by determining the effective concentration (EC50) (Figure 4) which corresponds to an absorbance equal to 0.5.
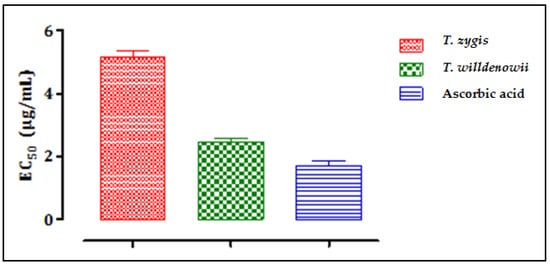

Figure 4. The effective concentrations (EC50) of T. zygis, T. willdenowii and ascorbic acid; data are mean ± SD, experiment was performed in minimum 2 replicates T. zygis: Thymus zygis, T. willdenowii: Thymus willdenowii.
From the results, we can deduce that the inhibition concentrations (IC50) to reduce 50% of the Fe3+ ions were 5.17 µg/mL, 2.46 µg/mL, and 1.76 µg/mL, for T. willdenowii, T. zygis, and ascorbic acid, respectively. There is a correlation between the chemical composition and the biological activity observed, especially with the high levels of carvacrol known for its high antioxidant potential. Indeed, several studies have demonstrated the superiority of the antioxidant power of essential oils with phenolic chemotypes (Carvacrol, thymol) [50][51][50,51]. Phenolics operate as reducing agents, hydrogen donors, and single oxygen donors due to their redox properties [52]. Nonetheless, other minor compounds can interact directly or in a synergistic or antagonistic manner to create a mixture endowed with more powerful activity. However, the antioxidant activity of the majority of compounds tested separately gives inferior results compared to the activity of the whole of the essential oil [53]. The two essential oils of the two thymes examined to reduce oxidation caused by free radicals demonstrated a potential to be effective against cancer and anti-infectious diseases and suggest that Thymus is a strong antioxidant that can be used as a natural antioxidant.
6. Antibacterial Activity of T. zygis and T. willdenowii EOs
6.1. Antibiotic Sensitivity Test
The antibiotic sensitivity profiles of the strains indicated in Table 4 and Table 5 were carried out according to the recommendations of the French Society of Microbiology and The European Committee on Antimicrobial Susceptibility Testing (EUCAST) [54]. From the antibiogram, we can conclude that Enterobacter cloacae exhibited resistance to Ticarcillin, Ofloxacin, Amoxicillin + Clavulanic acid, Colistin, and Amoxicillin. As for the Staphylococcu aureus, Salmonella Typhi, and Shigella dysenteriae strains, they were sensitive to all the antibiotics tested except E. coli which showed resistance to Ticarcillin and Cefalexin (Table 5). At the same time, Acinetobacter baumannii demonstrated complete resistance to all tested antibiotics. The strain A. baumanii is the most frequently encountered species in human infections. A study conducted on 754 strains, mainly from intensive care units (50.53%), showed very high resistance to beta-lactams (91%), Cefotaxime (50.3%), Ceftazidime and Imipenem (42.6%). Resistance to aminoglycosides ranged from 17.9% for netilmicin to 72.1% for gentamicin. Resistance to Ciprofloxacin was 65.8% and to trimethoprimesulfamethoxazole 75.8% [55]. This may be due to the higher resistance of Gram-negative bacteria due to the complexity of their cell wall, containing a double membrane in opposition to the single glycoprotein/teichoic acid membrane of Gram-positive bacteria ) [56]. The bacterial strains chosen for this research are of great interest in the clinical and health fields. Their increasing resistance to antibiotics has prompted further research into new and more effective natural products. Some strains of E. coli are virulent and can specifically trigger spontaneous infections of the gastrointestinal tract or urinary tract and even neonatal meningitis in humans or certain animal species. Other strains belonging to the symbiotic flora can also cause a variety of opportunistic infections, particularly in individuals with weakened immune defenses [56]. S. aureus is the cause of meningitis, osteomyelitis and diarrhea [57]. E. cloacae is a major pathogen within the genus Enterobacter. It is an opportunistic pathogenic Gram-negative bacillus mostly involved in nosocomial infections in compromised patients [58]. The bacterial pathogen S. typhi causes a serious systemic disease called typhoid, which is a major public health problem of global importance [59]. Shigellosis is an acute invasive intestinal infection caused by bacteria belonging to the genus Shigella; it is clinically manifested by diarrhea often bloody [60]. Antibiotic susceptibility testing was performed to demonstrate the power of the essential oils against resistant strains. The diameter of the inhibition zones was considered resistant for diameters less than 8 mm, and sensitive for those over 20 mm.
Table 4. Antibiotic sensitivity test for A. baumannii and E. cloacae.
| ATB | A. baumannii | ATB | E. cloacae |
|---|---|---|---|
| TIC75 | 6 ± 00 (R) | TIC75 | 6 ± 00 (R) |
| CAZ30 | 6 ± 00 (R) | CAZ30 | 20 ± 00 (S) |
| MEM10 | |||
| 6 ± 00 (R) | |||
| PRL | |||
| 75 | |||
| 6 ± 00 (R) | |||
S: sensitive at standard dose, R: resistant, ATB: antibiotics, TOB: Tobramycin, TIC: Ticarcillin, AML: Amoxicillin, FOX: Cefoxitin, CT: Colistin, CIP: Ciprofloxacin, AK: Amikacin, IPM: Imipenem, CAZ: Ceftazidime, PRL: Piperacillin, TE: Tetracycline, CN: Cefalexin, MEM: Meropenem, TIM: Ticarcillin + Clavulanic acid, OFX: Ofloxacin, Antibiotic disc load was in µg.
Table 5. Antibiotic sensitivity test for S. aureus, E. coli, S. Typhi, and S. dysenteriae.
| ATB | S. aureus | ATB | E. coli | S. | Typhi | S. dysenteriae | |||
|---|---|---|---|---|---|---|---|---|---|
| CIP5 | 23 ± 0.1 (S) | CT50 | 20 ± 0.1 (S) | 20 ± 0.1 (S) | 21 ± 0.2 (S) | ||||
| VA30 | 26 ± 0.3 (S) | MEM10 | 21 ± 0.2 (S) | 22 ± 0.2 (S) | 23.5 ± 0.1 (S) | ||||
| 6 ± 00 (R) | OFX5 | TE30 | 24.5 ± 0.2 (S)6 ± 00 (R) | ||||||
| TIC75 | 06 ± 00 (R) | 21.5 ± 00 (S) | 20.5 ± 0.3 (S) | TIM85 | 6 ± 00 (R) | AMC3 | 6 ± 00 (R) | ||
| CN15 | 21 ± 0.1 (S) | AK30 | 20 ± 0.1 (S) | 22 ± 0.2 (S) | 22 ± 0.1 (S) | IPM10 | 6 ± 00 (R) | IPM10 | 27 ± 0.1 (S) |
| MY15 | 30 ± 0.1 (S) | C30 | 27 ± 00 (S) | 29 ± 00 (S) | 20 ± 00 (S) | CT50 | 6 ± 00 (R) | CT50 | |
| E15 | 6 ± 00 (R) | ||||||||
| 20 ± 00 (S) | PRL75 | 21 ± 00 (S) | 21 ± 00 (S) | 21 ± 00 (S) | |||||
| CAZ30 | 22 ± 0.3 (S) | IPM10 | 23 ± 0.2 (S) | 21 ± 0.3 (S) | 23 ± 0.2 (S) | ||||
| TOB10 | 21.5 ± 0.1 (S) | CIP5 | 20 ± 0.1 (S) | 20.5 ± 0.2 (S) | 30.5 ± 0.1 (S) | ||||
| SXT25 | 20 ± 0.2 (S) | AMC30 | 21 ± 0.2 (S) | 21 ± 0.1 (S) | 20 ± 0.2 (S) | ||||
| FD10 | 23 ± 0.4 (S) | CN15 | 06 ± 00 (R) | 20 ± 0.2 (S) | 21 ± 0.1 (S) | ||||
| FOX30 | 22 ± 0.1 (S) | CAZ30 | 20 ± 0.1 (S) | 23.5 ± 0.1 (S) | 20.5 ± 0.2 (S) | ||||
| RD30 | 25 ± 00 (S) | CRO30 | 21 ± 00 (S) | 22 ± 00 (S) | 24 ± 00 (S) | ||||
| OFX5 | 20 ± 0.1 (S) | CTX30 | 20 ± 0.2 (S) | 21 ± 0.1 (S) | 23 ± 0.2 (S) |
S: sensitive at standard dose, R: resistant, ATB: antibiotics, CRO: Ceftriaxone, TOB: Tobramycin, AML: amoxicillin, FOX: Cefoxitin, C: Chloramphenicol, CT: Colistin, AMC: Amoxicillin + clavulanic acid, CIP: Ciprofloxacin, AK: Amikacin, IPM: Imipenem, CAZ: Ceftazidime, PRL: Piperacillin, SXT: Trimethoprim + sulfamethoxazole, TE: Tetracycline, CN: Cefalexin, MEM: Meropenem, TIM: Ticarcillin + clavulanic acid, OFX: Ofloxacin, VA: Vancomycin, MY: Lincomycin, FD: Fusidic acid, RD: Rifampicin, CTX: Cefotaxime. Antibiotic disc load was in µg.
6.2. Antibacterial Activity of T. zygis and T. willdenowii Essential Oils
The antibacterial activity of T. zygis and T. willdenowii EOs was evaluated by the disk diffusion method and by tests to determine the MIC and MBC. For the disk diffusion method, EOs are considered to be active when they induce an inhibition zone greater than or equal to 12 mm [61][62][61,62]. The average inhibition diameters, generated by the EOs tested on the different bacterial strains tested were presented in Table 6 given below. Statistical analysis of the results showed that the diameters of inhibition were significantly different for EOs (p < 0.05). According to the antibacterial results tests, all the bacteria demonstrated significant inhibition zones when tested against the two EOs. T. zygis EO exhibited the most powerful activity against all the studied bacteria, while that of T. willdenowii recorded moderate activities against S. dysenteriae and S. Typhi. With an inhibition diameter between 75 mm and 84 mm for a concentration of 2 µL/mL up to 12 µL/mL, S. aureus was shown to be the most sensitive to T. zygis EO. In addition, Gram-positive bacteria are more sensitive to T. zygis essential oil action compared to Gram-negative bacteria. It is known that the structure of the cell wall of Gram-positive bacteria makes them vulnerable to the action of essential oils [4]. These different results between the two essential oils could be explained by the bioactivity of the chemical compounds of each oil, the functional groups of the major compound (alcohols, phenols, aldehydes), and the synergistic effects between the components. Thus, the most effective chemical compounds which have a broad spectrum of antimicrobial action are phenols (Thymol, carvacrol, and eugenol), alcohols (α-terpineol, terpinene-4-ol, menthol, geraniol, linalool), aldehydes (geraniol, citral, and neral), and ketones (carvone, pulegone, and camphor) [63][64][63,64]. Analysis of the results related to the MIC and MBC of the two EOs revealed their great bactericidal power (Table 7). Nonetheless, Gram-positive bacteria were found to be susceptible to EOs as well as Gram-negative with a difference between MIC and MBC. Indeed, E. coli was shown to be sensitive from the concentration of 2 μL/mL towards the EO of T. zygis whereas S. dysenteriae was resistant up to the concentration of 10 μL/mL of T. willdenowii EO. The chemotype carvacrol found in T. zygis essential oil with a concentration of 52.2% of the total essential oil remains the most effective compared to the other T. willdenowii essential oil which contains only 16.2% of carvacrol. The high carvacrol content of T. zygis essential oil could explain the bactericidal effect on the different strains. Phenols are, due to the acidic nature of their hydroxyl substituent, considered as the most active compounds on bacteria [65] knowing that the total chemical composition of the EO of this thyme is dominated by 69.53% of alcohols. Indeed, alcohols are particularly active against bacterial strains, because they are soluble in aqueous media and cause significant damage to the cell walls of microorganisms [66]. Alcohols have bactericidal rather than bacteriostatic activity [67].
Table 6. Antibacterial activity of T. zygis and T. willdenowii EOs.
| Essential Oils Concentration Tested (µL/mL) | Essential Oils Inhibition Diameter (mm) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. zygis | T. willdenowii | S. aureus | E. coli | S. | Typhi | A. baumannii | E.cloacae | S. dysenteriae | |||||||||
| Tz | |||||||||||||||||
| MIC µL/mL | Tw | Tz | Tw | Tz | Tw | Tz | Tw | Tz | Tw | Tz | Tw | ||||||
| 06 ± 0.008* | |||||||||||||||||
| 06 ± 0.005 * | |||||||||||||||||
| S. dysenteriae | 06 ± 0.0001 * | 06 ± 0.0003 * | 10 ± 00 | ns | 10 ± 00 | ns | |||||||||||
The experiment was performed in minimum 2 replicates with ** p ≤ 0.01, * p ≤ 0.05; ns: not significant.
7. Antifungal Activity of T. zygis and T. willdenowii EOs
7.1. Sensitivity of Fungal Strains
The antifungal susceptibility profiles of the strains indicated in Table 8 were carried out according to the recommendations of EUCAST. Both strains C. glabrata and C. albicans were sensitive to the antifungal agent (Fluconazole) while Candida spp., A. fischeri, and F. solani were resistant. The candidas particularly responsible for the infection, which typically occurs in patients with impaired immune function or who have had a mucosal invasive procedure (ANOFEL, 2014). The prevalence of yeasts of the genus Candida resistant to first-generation triazole antibiotics is low. Primary resistance has been described in less than 2.5% of cases for fluconazole and in less than 9% of cases for litraconazole (5). C. albicans is most often susceptible, C. glabrata is often susceptible-dose dependent [68]. Whereas fluconazole is not active in vitro against filamentous fungi such as Aspergillus spp. (MIC of 64 mg/L) [69]. Fungi of the genus Fusarium are resistant to the majority of available human antifungal agents: they are resistant in vitro to flucytosine, to first-generation triazoles (fluconazole, itraconazole) [70].
Table 8. Susceptibility test of fungal strains to antifungal.
| Fungal Species | Antifungal: Fluconazole V = 20 µL | |||||
|---|---|---|---|---|---|---|
| Fungal Species |
T. zygis | T. willdenowii | ||||
| MBC µL/mL | MIC µL/mL | MBC µL/mL | ||||
| 2 | 75 ± 00 *** | 33 ± 0.2 *** | 54 ± 00 *** | |||
| C. glabrata | S | |||||
| C. albicans | S | |||||
| Candida | spp. | R | ||||
| A. fischeri | R | |||||
| F. solani | R | |||||
7.2. Antifungal Activity of Essential Oils
The genus Thymus was used against bacteria and fungi in the traditional pharmacopeia [71]. The volatile oils studied showed very good antifungal power, referring to the reading established by Meena and Sethi, and Ponce et al. [72][73][72,73]. The diameter of the inhibition zone is noted as resistant for diameters less than 8 mm, and sensitive for diameters greater than 20 mm. From Table 9 it can be seen that the EO of T. zygis showed the best inhibition diameters compared to the control (fluconazole) while T. willdenowii demonstrated a similar (F. solani) and even better inhibition zone (A. fischeri) when also compared to fluconazole the molds and yeasts showed a sensitivity to the concentration of 20 µL of the two Eos and the inhibition zones were of the order of 40 mm for C. albicans, 29 mm for C. glabrata, 27 mm for Candida spp., 18 mm for F. solani and 40 mm for A. fischeri, when using T. zygis EO, all diameters were greater than the control values used (fluconazole). The EO of T. willdenowii, when used, recorded smaller diameters than the other EO (T. zygis) (Table 9 and Figure 5). Regarding the MIC and MFC (Table 10), the Eos of the two thymes showed significant fungicidal activity on both yeasts and molds, even against strains resistant to the control, Candida spp., A. fischeri and F. solani. The inhibitory effect of T. zygis EO was manifested at 20 µL/mL while that of T. willdenowii was active at 30 µL/mL. MFC/MIC values are equal to 1.


Figure 5. Antifungal activity of the essential oil of T. willdenowii tested on C. albicans (L13), C. glabrata (L14), Candida spp. (L15), F. solani (H13), and A. fischeri (G2).
Table 9. Effect of the EO on the growth of yeasts and molds.
| Species | C = 20 µL/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile Oil Inhibition Diameter (mm) | |||||||||||
| MIC µL/mL | MFC µL/mL | MFC/MIC | MFC µL/mL | MFC µL/mL | MFC/MIC | C. glabrata | C. albicans | Candida | spp. | F. solani | A. fischeri |
| 15 ± 00 | ns | 20 ± 00 * | 06 ± 00 | ns | 71.5 ± 0.1 *** | 30 ± 00 ** | 60.1 ± 0.1 *** | 14 ± 00 | ns | 6 ± 00 | |
| E. coli | ns | 02 ± 0.002 * | 6 ± 00 | ||||||||
| Refractive index | 1.50 ± 0.05 | 1.33 ± 0.04 | |||||||||
| Degree degree (%) | 85.44 ± 0.05 | 76.62 ± 0.05 | |||||||||
4. Chemical Composition of T. zygis and T. willdenowii Essential Oils
Figure 1A,B and Table 3 represent the chromatograms and the details of the composition of the two EOs with their intensities. A total of 31 compounds were identified in the EO of T. zygis (sum of approximately 99.84%), while 33 compounds were identified in that of T. willdenowii (sum of approximately 98.69%). The chemical composition of T. zygis EO consists mainly of oxygenated monoterpenes (68.7%) and hydrocarbon monoterpenes (27.55%) with carvacrol (52.20%), o-cymene (23.14%), and thymol (9.68%) as major compounds, accompanied by other compounds at relatively low levels (borneol (3.30%), linalool (2.40%), γ-terpinene (1.98%) and caryophyllene oxide (1.06%)). Analyzing further the chemical composition of this EO, six families were noted: Alcohols (69.53%), hydrocarbons (28.85%), epoxides (1.06%), ketones (0.24%), aldehyde (0.25%) and ethers (0.07%) (Figure 2). By comparing the results to T. zygis EOs from other regions of Morocco and around the world, chemical composition differences can be seen. Indeed, in Morocco, the essential oil of the same species of Krouchen, (Middle Atlas of Morocco) was dominated mainly by thymol (33.02%), o-cymene (32.02%) and (E)-β-ocimene (11.90%) [26]. In the Aknoul region (Taza region), the chemical composition was marked by the presence of thymol (37.5%), γ-terpinene (29.7%), and p-cymene (12.1%) [27]. In Europe, T. zygis from Northern Portugal was mainly composed of thymol (23.8%), geraniol (18.2%), geranyl acetate (16.3%) and p-cymene (13.6%) [28]. The essential oils of several samples of T. zygis from Spain studied by Richard et al. (1985), consist mainly of thymol (1.1 to 30.7%) or carvacrol (6.5 to 42.9%) accompanied by other constituents such as p-cymene (23.3 to 28.5%), caryophyllene oxide (1.5 to 9.8%), linalool (1.5 to 4%) and thymol methyl ether (0 to 4.5%) [8]. Older studies on the subspecies gracilis revealed the presence of phenolic chemotypes, mainly thymol or carvacrol [29][30][31][32][33][34][29,30,31,32,33,34]. We can conclude that the EO of T. zygis was characterized by a very interesting chemical composition. Concerning the EO of T. willdenowii, the chemical composition was marked by, oxygenated monoterpenes (32.81%), hydrocrboned sesquiterpene (31.61%), oxygenated sesquiterpene (13.35%), and hydrocarbon monoterpenes (8.95%), with the dominance of carvacrol (16.19%), geranyl acetate (8.35%), caryophyllene oxide (6.90%), camphor (5.99%), and (E)-caryophyllene (5.59%), in addition to other compounds at relatively low percentages such as borneol (4.74%) and β-elemene (3.96%). Comparing our findings with those of other researchers, we found that the EO collected from the Col du Zad in the region of Khenifra was rich in compounds other than monoterpenes such as terpenyl acetate (26.99%). T. willdenowii EO collected in Annzala of the region of Midelt at an altitude of (1605 m) was rich in oxygenated monoterpenes represented by camphor (24.99%) [18].
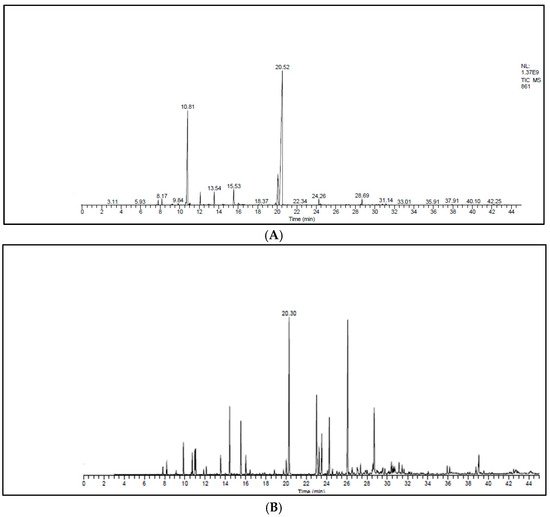
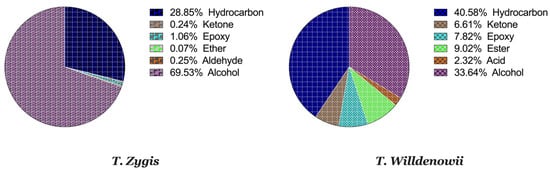

Figure 1. Chromatograms of the EOs. (A) T. zygis, (B) T. willdenowii.

Figure 2. Percentage of chemical families in the thymes EO.
Table 3. Chemical composition of the thymes EOs.
| No. | Compounds | Kováts Index (KI) | Molecular Formula | Area% | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. willdenowii | T. zygis | ||||||||||||||||||||||||||||
| 1 | α | -Pinene | 939 | ||||||||||||||||||||||||||
| T. zygis | 40 ± 0.3 * | 29 ± 0.5 | C | 10 | H | 16 | 0.59 | 0.62 | |||||||||||||||||||||
| ns | |||||||||||||||||||||||||||||
| 02 ± 0.001 * | 04 ± 0.006 * | 04 ± 00 | ns | ns | |||||||||||||||||||||||||
| C. glabrata | 20 ± 0.001 * | 20 ± 0.0009 * | 27 ± 1.2 | ns | 18 ± 1.3 | ns | 140 ± 2.1 ** | ||||||||||||||||||||||
| 2 | Camphene | 954 | |||||||||||||||||||||||||||
| 30 ± 00 | ns | 30 ± 00 | ns | 1 | 4 | 84 ± 0.2 *** | 33.8 ± 0.1 *** | 60 ± 00 *** | |||||||||||||||||||||
| S. aureus | 02 ± 0.0009 * | C | 10 | H | 16 | 0.96 | 0.78 | ||||||||||||||||||||||
| 16.3 ± 0.1 | ns | 24.5 ± 0.3 * | 02 ± 0.004 * | 04 ± 00 | ns | 6 ± 00 | ns | 72 ± 00 *** | 35 ± 00 ** | 64.5 ± 0.4 *** | T. willdenowii | 17 ± 0.1 | ns | 19 ± 1.1 15.5 ± 1.2 | ns | ns | 23 ± 0.1 18 ± 0.1 | ns | ns | 12 ± 00 6 ± 00 | ns | ns | 22 ± 00 * | 3 | |||||
| 04 ± 0.01 ** | β-Pinene | 979 | C | 10 | H | 16 | - | 0.06 | |||||||||||||||||||||
| 6 | 84 ± 0.1 *** | 38 ± 0.3 *** | 71 ± 00 *** | 18 ± 00 * | 30.1 ± 0.2 ** | 13.5 ± 0.1 | ns | 76.3 ± 0.2 *** | 43.6 ± 0.1 *** | 71.3 ± 0.3 *** | 21 ± 00 * | 4 | 1-Octen-3-ol | 979 | C | 8 | H | 16 | O | - | 0.20 | ||||||||
| S | . Typhi | 04 ± 0.003 * | 04 ± 00 | ns | |||||||||||||||||||||||||
| Fluconazole | 06 ± 00 | ns | 06 ± 00 | 37.5 ± 0.9 ** | 6 ± 00 | ns | |||||||||||||||||||||||
| 24.7 ± 0.1 | ns | 23.7 ± 0.2 | 24 ± 0.6 | 16.3 ± 0.3 | 13.7 ± 02 | 8 | 84 ± 0.6 *** | 42 ± 00 *** | 82.2 ± 0.2 *** | 21.4 ± 0.2 * | 50 ± 00 *** | 16 ± 00 | ns | 78.1 ± 0.1 *** | 51 ± 00 *** | 77 ± 00 *** | 23 ± 00 * | 40 ± 00 *** | 13 ± 00 | ns | 5 | Myrcene | 990 | C | 10 | H | 16 | 2.43 | 0.27 |
| 10 | 84 ± 0.3 *** | 47.2 ± 0.1 *** | 84 ± 00 *** | 22.1 ± 0.3 * | 52 ± 00 *** | 18.9 ± 1.3* | 80.7 ± 1.4 *** | 56 ± 00 *** | 78.9 ± 0.1 *** | 35 ± 00 * | 48.6 ± 0.4 *** | 15 ± 00 | ns | 6 | 3-δ-Carene | 1002 | C | 10 | H | 16 | - | 0.29 | |||||||
| 7 | p | -Cymene | |||||||||||||||||||||||||||
| 12 | 84 ± 00 *** | 48 ± 00 *** | 84 ± 00 *** | 23 ± 00 * | 57.5 ± 00 *** | 23 ± 00 * | 81 ± 00 *** | 60 ± 00 *** | 82 ± 00 *** | 38 ± 00 * | 51 ± 00 *** | 18 ± 00 * | 1024 | C | TOB10 | 10 | |||||||||||||
The experiments were performed in a minimum of 2 replicates with ** p ≤ 0.01, * p ≤ 0.05; ns: not significant compared to fluconazole.
Table 10. MIC and MFC values of T. zygis and T. willdenowii essential oils.
| C. albicans | 20 ± 0.015 ** | 20 ± 0.0001 * | 1 | 20 ± 0.0009 * | 20 ± 00 | ns | 1 | |||||||||||||||||||
| Candida | spp. | 20 ± 00 | ns | 20 ± 00 | ns | 1 | 20 ± 0.002 * | 20 ± 0.007 * | 1 | |||||||||||||||||
| F. solani | 30 ± 00 | ns | 30 ± 0.0002 * | 1 | 30 ± 00 | ns | 30 ± 00 | ns | ||||||||||||||||||
| 6 ± 00 (R) | ||||||||||||||||||||||||||
| H | FOX30 | 14 | 1.78 | - | ||||||||||||||||||||||
| 21 ± 00 (S) | ||||||||||||||||||||||||||
| 1 | ||||||||||||||||||||||||||
| A. fischeri | 20 ± 0.014 ** | 20 ± 00 | ns | 1 | 20 ± 00 | ns | 20 ± 00 | ns | 1 | 2(DMSO) | 06 ± 00 | 06 ± 00 | 06 ± 00 *** | 06 ± 00 | 06 ± 00 | 06 ± 00 | 8 | o | -Cymene | 1026 | C | 10 | H | 14 | - | 23.14 |
| 9 | Limonene | 1029 | C | 10 | H | 16 | 2.16 | 0.28 | ||||||||||||||||||
| 10 | 1,8-Cineole | 1031 | C | 10 | H | 18 | O | 1.77 | 0.18 | |||||||||||||||||
| 06 ± 00 | 11 | (Z)- | β-ocimene | 1037 | C | 10 | H | 16 | 0.42 | - | ||||||||||||||||
| 06 ± 00 | 12 | γ | -Terpinene | 1059 | C | 10 | H | 16 | 0.61 | 1.98 | ||||||||||||||||
| 13 | Cis | -Linalool oxide | 1072 | C | 10 | H | 18 | O | 2 | - | 0.13 | |||||||||||||||
| 14 | Trans | -Linalool oxide | 1086 | C | 10 | H | 18 | O | 2 | - | 0.25 | |||||||||||||||
| 06 ± 00 | 06 ± 00 | 06 ± 00 | 06 ± 00 | CIP5 | 6 ± 00 (R) | AML10 | 6 ± 00 (R) | 15 | ||||||||||||||||||
| TE30 | Linalool | 1096 | C | 10 | H | 18 | O | 2 | 1.78 | 2.40 | ||||||||||||||||
| 6 ± 00 (R) | 16 | Camphor | 1146 | C | 10 | H | 16 | O2 | 5.99 | 0.16 | ||||||||||||||||
| CN15 | 17 | Borneol | 1169 | C | 10 | H | 18 | O | 4.74 | 3.30 | ||||||||||||||||
| 22 ± 0.2 (S) | 18 | Terpinen-4-ol | 1177 | C | 10 | H | 18 | O | 1.06 | 0.35 | ||||||||||||||||
| 19 | α-Terpineol | 1188 | C | 10 | H | 18 | O | - | 0.10 | |||||||||||||||||
| 20 | Pulegone | 1237 | C | 10 | H | 16 | O | - | 0.08 | |||||||||||||||||
| 21 | Carvacrol methyl ether | 1244 | C | 10 | H | 16 | O | - | 0.07 | |||||||||||||||||
| 22 | Thymol | 1290 | ||||||||||||||||||||||||
| CN15 | 21.5 ± 0.1 (S) | AK30 | C | 10 | H | 14 | O | 1.28 | 9.68 | |||||||||||||||||
| 19 ± 00 (I) | 23 | Carvacrol | 1299 | C | 10 | H | 14 | O | 16.19 | 52.2 | ||||||||||||||||
| 24 | Geranyl acetate | 1381 | C | 12 | H | 20 | O | 2 | 8.35 | - | ||||||||||||||||
| 25 | β-Bourbonene | 1388 | C | 15 | H | 24 | 2.48 | - | ||||||||||||||||||
| 26 | β-Elemene | 1390 | C | 15 | H | 24 | 3.96 | - | ||||||||||||||||||
| 27 | ( | E | )-Caryophyllene | 1419 | C | 15 | H | 24 | 5.59 | 0.99 | ||||||||||||||||
| 28 | β-YLangene | 1420 | C | 15 | H | 24 | 0.51 | - | ||||||||||||||||||
| 29 | γ-Elemene | 1436 | C | 15 | H | 24 | 0.82 | - | ||||||||||||||||||
| 30 | Germacrene D | 1481 | C | 15 | H | 24 | 16.51 | - | ||||||||||||||||||
| AK | 31 | α-Murolene | 1500 | C | 15 | H | 24 | - | 0.09 | |||||||||||||||||
| 32 | γ-Amorphene | 1512 | C | 15 | H | 24 | 0.87 | 0.07 | ||||||||||||||||||
| 33 | γ-Cadinene | 1513 | C | 15 | H | 24 | 0.87 | - | ||||||||||||||||||
| 34 | Spathulenol | 1578 | C | 15 | H | 24 | O | 0.96 | 0.18 | |||||||||||||||||
| 35 | Caryophyllene oxide | 1583 | C | 15 | H | 24 | O | 6.90 | 1.06 | |||||||||||||||||
| 36 | Allo | -Aromadendrene epoxide | 1640 | C | 15 | H | 24 | O | 0.92 | - | ||||||||||||||||
| 37 | Caryophylla-4(12),8(13)-dien-5β-ol | 1640 | C | 15 | H | 24 | O | - | 0.09 | |||||||||||||||||
| 38 | Epi | -α-Cadinol | 1640 | C | 15 | H | 26 | O | - | 0.09 | ||||||||||||||||
| 39 | Cubenol | 1646 | C | 15 | H | 26 | O | |||||||||||||||||||
| 30 | 0.55 | - | ||||||||||||||||||||||||
| 40 | Eudesmol | 1650 | C | 15 | H | 26 | O | 0.61 | - | |||||||||||||||||
| 41 | Cedr-8(15)-en-10-ol | 1652 | C | 15 | H | 24 | O | - | 0.20 | |||||||||||||||||
| 42 | α-Cadinol | 1654 | C | 15 | H | 18 | 1.32 | - | ||||||||||||||||||
| 43 | Cadalene | 1676 | C | 15 | H | 18 | - | 0.23 | ||||||||||||||||||
| 44 | Germacra-4(15),5,10(14)-trien-1α-ol | 1686 | C | 15 | H | 24 | O | 2.09 | - | |||||||||||||||||
| 45 | hexahydrofarnesyl acetone |
The experiment was performed in minimum 2 replicates with *** p ≤ 0.001, ** p ≤ 0.01, * p ≤ 0.05; ns: not significant compared to control DMSO. Tz: T. zygis; Tw: T. willdenowii.
Table 7. Determination of MIC and MBC of T. zygis and T. willdenowii EOs.
| Bacteria | |||
|---|---|---|---|
| A. baumannii | |||
| 02 ± 0.001 * | 02 ± 0.001 * | 04 ± 0.001 * | 04 ± 0.012 ** |
| E. cloacae | 02 ± 0.007 * | 02 ± 00 | ns |
The experiment was performed in minimum 2 replicates with ** p ≤ 0.01, * p ≤ 0.05; ns: not significant.
This great activity can be linked to the presence of predominant phenolic compounds such as carvacrol which is known for its antimicrobial properties [74][75][74,75]. The mechanism of action of Eos remains controversial; some studies suggest that these components can enter the microorganism and react with active sites of enzymes and or interfere with cell metabolism, but several proposals lean towards disruption of cell membranes and pro-oxidant cytotoxic effects [76]. Essential oils’ action is frequently affected by their hydrophobic characteristic, which enables them to permeate the bacterial cell membrane’s phospholipidic double layer. This may cause a change in membrane conformation, a chemiosmotic disturbance, and ion leakage (K+) [77].
Some essential oil phenolic compounds interact with membrane proteins of microorganisms, such as the ATPase enzyme, either directly on the hydrophobic portion of the protein or by interfering with protons translocation through the membrane, inhibiting the phosphorylation of ADP. Decarboxylation of amino acids in E. aerogenes has also been shown to be inhibited [78]. The results showed that the inhibition diameters are significantly different for EOs (p < 0.05).
Because of its anti-infectious properties, the EO of the two examined thymes may be likened to antibiotics and antifungals. This antimicrobial action on yeasts, as well as Gram (+) and Gram (−) bacteria that are multi-resistant to antibiotics, may help in the battle against infectious illnesses and could lead to the use of these EOs in the pharmaceutical and agrifood industries.
