Obesity is a disease characterized by an excessive body fat accumulation and by the presence of a subclinical chronic inflammation. It is related to many comorbidities, such as non-alcoholic fatty liver disease (NAFLD), the predominant cause of chronic liver disease in many parts of the world. NAFLD is a disease spectrum which starts with simple steatosis (the accumulation of fat in the liver) and could progress to steatohepatitis, cirrhosis or even hepatocarcinoma, mainly due to sedentary lifestyle.
Gut microbiota is a metabolic organ involved in physiological homeostasis and is defined as all the microorganisms that habit along the digestive tract. The alteration of its composition and functionality, called dysbiosis, has been associated with many pathologies, such as obesity and non-alcoholic fatty liver disease (NAFLD) development. Gut microbiota emerges as a therapeutic target, in which probiotics or prebiotics play a central role. Probiotics are live microorganisms that have beneficial effects on health status when are consumed in proper doses, whereas prebiotics are non-digestible ingredients which promote the growth of beneficial microorganisms in the gut. A synbiotic is a combination of prebiotics and probiotics that confers a healthy benefit on the host.
- Akkermansia muciniphila
- childhood obesity
- gut microbiota
- quercetin
- synbiotic
1. Introduction
Nowadays, obesity is a worldwide epidemic with a 13% of prevalence in adults and with a rising trend in children and adolescents [1]. In its multifactorial pathogenesis, the sedentary patterns and the unhealthy habits have an important role. Moreover, obesity is associated with many comorbidities, such as non-alcoholic fatty liver disease (NAFLD) [2], one of the commonest manifestations of chronic hepatic disease with a worldwide prevalence of 20–30% [3]. The pathogenesis of NAFLD, according to the multiple-hit hypothesis [4], is explained by the concurrent action of many factors. In the last few years, gut microbiota has been identified as a key factor in NAFLD and obesity development [5][6][7]. Gut microbiota is a metabolic organ with an essential role in human homeostasis, which carries out many indispensable functions such as fermentation of carbohydrates and its transformation in short-chain fatty acids (SCFAs) and transformation of primary bile acids (BAs) into secondary metabolites [8].
Currently, the main strategy to manage NAFLD and obesity is to change lifestyle towards healthy patterns [3]. However, obesity-related NAFLD patients usually have a low adherence to these interventions, being necessary to search for new strategies in the treatment of these pathologies [9][10]. Due to the role of gut microbiota alterations in the development of NAFLD and obesity, the administration of probiotics or prebiotics are reasonable therapies in the treatment of these diseases. Additionally, the combination of both, namely synbiotics, may potentiate the efficacy of the intervention [11][12]. The flavonoid quercetin, with antioxidant and anti-inflammatory properties, has been reported to counteract NAFLD in in vivo models through a possible and additional prebiotic effect [7][13]. Moreover, the bacteria Akkermansia muciniphila has been pointed out as a potential probiotic due to its protective effect in obesity development [14][15][16].
Therefore, the aim of this study is to evaluate the combinatory effect of a nutritional intervention together with quercetin supplementation and A. muciniphila administration on early obesity and NAFLD development in an in vivo model.
2. Methods
To carry out this study, as it is shown in Figure 1, 21-day-old Wistar rats were fed with control or high fat diet (HFD) for 6 weeks. After that, blood and faecal samples were collected and all animals were fed with control diet supplemented with quercetin, A. muciniphila or the combination of both for 3 weeks. Finally, animals were sacrificed and samples were collected. Histopathological and biochemical parameters as well as gut microbiota composition were analyzed. Moreover, plasma BAs quantification was carried out.
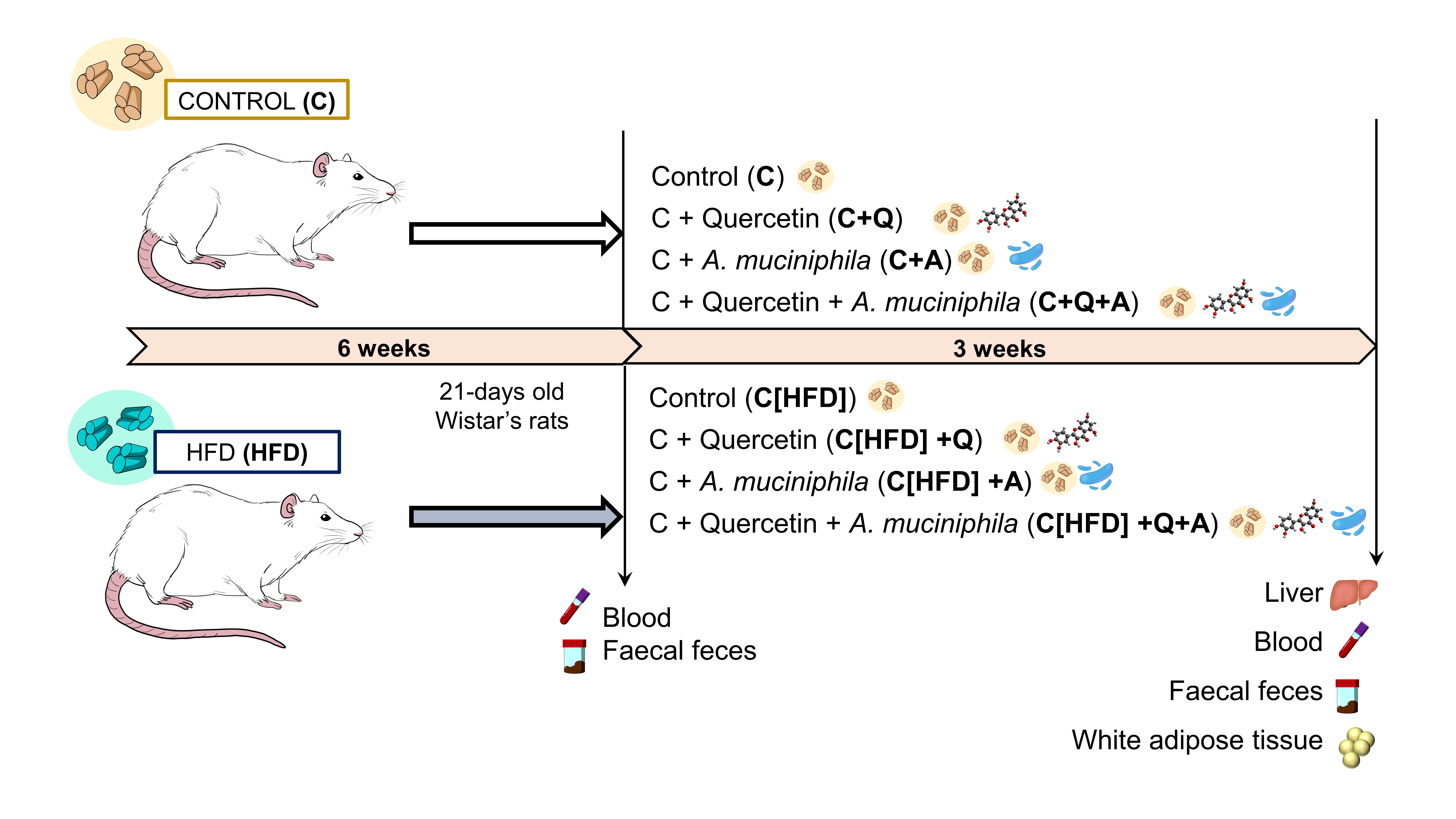 Figure 1. Experimental design.
Figure 1. Experimental design.
3. Results and Discussion
After 6 weeks of HFD feeding, juvenile rats manifested higher body weight and food intake in comparison with the control group. Besides, biochemical analysis showed important alterations in plasma such elevated alanine aminotransferase (ALT), cholesterol and incremented insulin concentration and HOMA‐IR index, which is closely linked to hepatic insulin resistance in NAFLD [17].
Moreover, 6-week HFD fed rats presented lower concentration of total bacteria DNA and a modified gut microbiota profile not only at phylum level, but also at class and genus level, highlighting higher abundance of Lactobacillus and Blautia in contrast with control group. In this sense, high levels of Lactobacillus has been identified in obesity and NAFLD [5][18][19], whereas Blautia increasement has been related with NAFLD in pediatric patients [20].
After 3 weeks of dietary intervention and administration of A. muciniphila and quercetin, the synbiotic reduced the concentration of fasting blood glucose, plasma insulin concentration and HOMA-IR index observed at 6 weeks of HFD intake, remarking their beneficial effects to counteract insulin resistance as previously described [7][15]. Also, reduced white adipose tissue and higher leptin concentration in plasma were observed after synbiotic administration. In absence of insulin resistance or metabolic disorders, high leptin concentration could promote energy expenditure and control food intake to compensate the metabolic disorders caused by HFD diet [21].
Regarding white adipose tissue status, relative expression of Ppary and Plin2 were reduced after the synbiotic, which has been associated to a protective role against insulin resistance and obesity and NAFLD development [22][23]. Additionally, the relative expression of Cebpa, Dgat2 and Srebp2 involved in the novo lipogenesis were upregulated by HFD-diet and this increment was counteracted by the synbiotic administration [17][24]. Moreover, Pparα upregulation, which modulates lipid metabolism and has a role in fatty acid beta-oxidation and regulation of their circulation [25][26], may result in a decrease of triglyceride synthesis and NAFLD progression. Regarding inflammation status, a protective role of the synbiotic administration was observed in the reduced relative expression of proinflammatory cytokines (Tlr2, Il6 and Il1β), confirming the effect of quercetin or A. muciniphila supplementation previously reported [7][15].
Beneficial effects of the synbiotic were also reflected in the gut microbiota composition. At phylum level, the relative abundance of Cyanobacteria was increased after synbiotic administration whereas Actinobacteria, a common marker of obesity [27], was decreased. At genus level, Blautia (highly detected in NAFLD adult and pediatric patients [20][28]) and Coprobacillus (previously associated with HFD [29]) were reduced in all quercetin-supplemented experimental groups. Besides, after synbiotic administration, Lactobacillus and Lactococcus, which correlate positively with fasting plasma insulin [28][30], reduced their relative abundance. On the contrary, Oscillospira, which negatively correlates with hepatic fat deposition and less expression in NAFLD and obese patients [31][32], was increased in both control and HFD groups. All of these results support the capacity of the synbiotic on gut microbiota reshaping and its beneficial effect counteracting obesity and NAFLD.
Also, changes in gut microbiota composition caused by the synbiotic promote beneficial modifications in bile acid (BA) composition, synthesis and transport in the liver. The intervention along with A. muciniphila and quercetin increased the total plasma BA pool mainly primary and unconjugated hydrophilic BAs, establishing a healthier hydrophilic BA profile [33]. Moreover, the increased gene expression of BA synthesis genes such as Cyp7a1 and Cyp8b1 and BA transport genes like Nctp, Bsep, Mdr2 and Mrp2 suggested an enhancement on BAs synthesis and bile flux linked to the synbiotic administration.
All these results were subjected to a correlation analysis, showing a whole network in which the synbiotic seems to induce changes in gut microbiota, that in turn are related to the improvement observed in our in vivo model.
In conclusion, the modulation of the BA profile, the upregulation of its synthesis and transport flux, as well as a modulation in hepatic inflammatory status and lipogenesis process in white adipose tissue could denote innovative mechanisms behind the beneficial effects of A. muciniphila in metabolic disease and support its use in combination with quercetin as a possible treatment of NAFLD and obesity development (Figure 2).
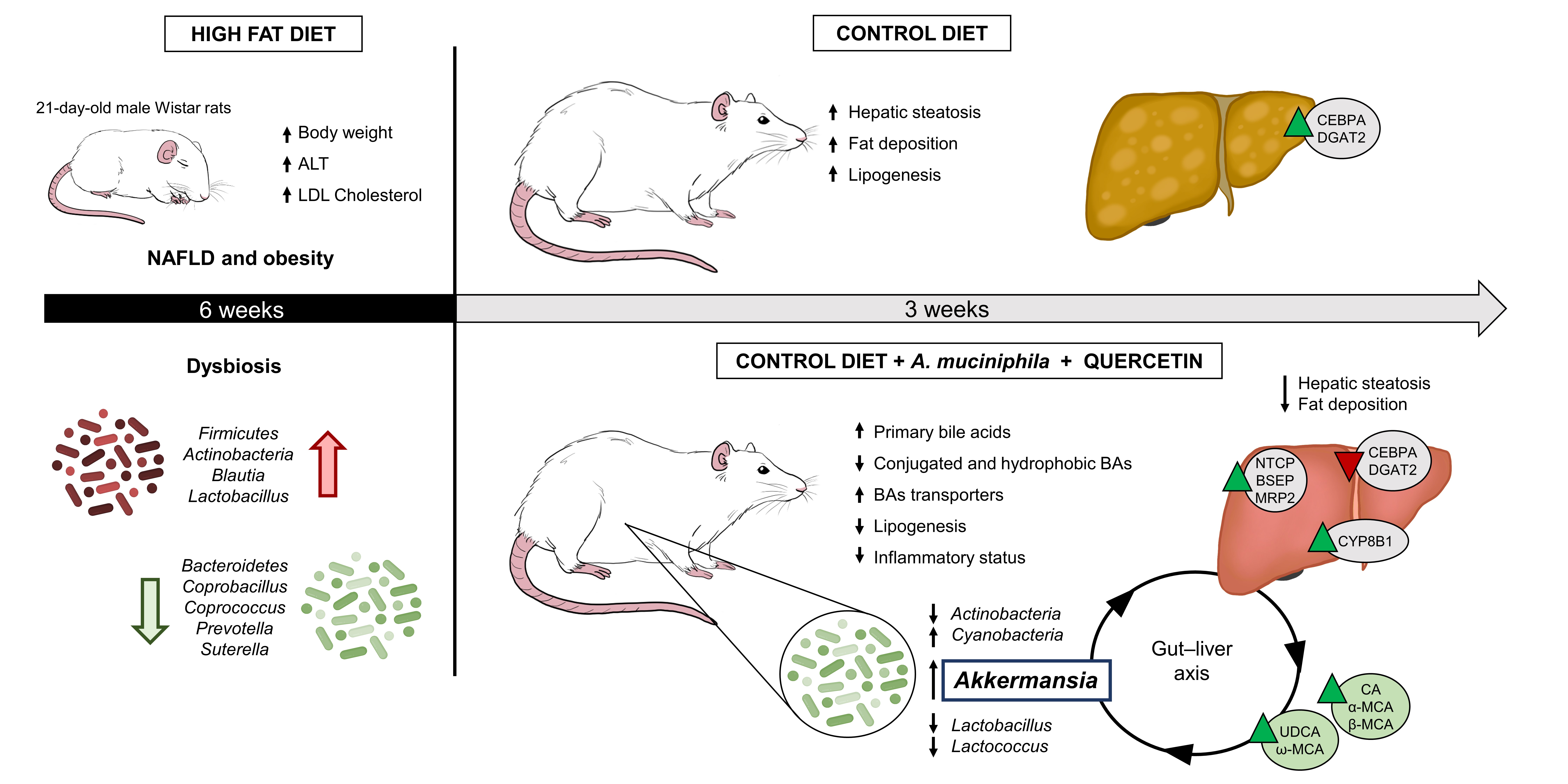 Figure 2. Graphical abstract.
Figure 2. Graphical abstract.
This entry is adapted from: https://doi.org/10.3390/antiox10122001
References
- Obesity and overweight. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 October 2021)
- Schwenger, K.J.P.; Bolzon, C.M.; Li, C.; Allard, J.P. Non-alcoholic fatty liver disease and obesity: The role of the gut bacteria. Eur. J. Nutr. 2019, 58, 1771–1784, doi:10.1007/s00394-018-1844-5.
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97, doi:10.1016/j.metabol.2018.11.014.
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846, doi:10.1002/hep.24001.
- Juárez-Fernández, M.; Porras, D.; García-Mediavilla, M.V.; Román-Sagüillo, S.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Aging, gut microbiota and metabolic diseases: Management through physical exercise and nutritional interventions. Nutrients 2021, 13, 16, doi:10.3390/nu13010016.
- Alagiakrishnan, K.; Halverson, T. Holistic perspective of the role of gut microbes in diabetes mellitus and its management. World J Diabetes 2021, 12, 1463–1478, doi:10.4239/WJD.V12.I9.1463.
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202, doi:10.1016/j.freeradbiomed.2016.11.037.
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39.
- Panera, N.; Barbaro, B.; Della Corte, C.; Mosca, A.; Nobili, V.; Alisi, A. A review of the pathogenic and therapeutic role of nutrition in pediatric nonalcoholic fatty liver disease. Nutr. Res. 2018, 58, 1–16, doi:10.1016/j.nutres.2018.05.002.
- Vittorio, J; Lavine, J.E. Recent advances in understanding and managing pediatric nonalcoholic fatty liver disease. F1000Research 2020, 9, 377.
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158.
- López-Almela, I.; Romaní-Pérez, M.; Bullich-Vilarrubias, C.; Benítez-Páez, A.; Gómez Del Pulgar, E.M.; Francés, R.; Liebisch, G.; Sanz, Y. Bacteroides uniformis combined with fiber amplifies metabolic and immune benefits in obese mice. Gut Microbes 2021, 13, 1–20, doi:10.1080/19490976.2020.1865706.
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Olcoz, J.L.; Jover, R.; Jorquera, F.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Functional interactions between gut microbiota transplantation, quercetin, and high-fat diet determine non-alcoholic fatty liver disease development in germ-free mice. Mol. Nutr. Food Res. 2019, 63, e1800930, doi:10.1002/mnfr.201800930.
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016, 23, 107–113, doi:10.1038/nm.4236.
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103, doi:10.1038/s41591-019-0495-2.
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125, doi:10.1111/1751-7915.13410.
- Badi, R.M.; Mostafa, D.G.; Khaleel, E.F.; Satti, H.H. Resveratrol protects against hepatic insulin resistance in a rat’s model of non-alcoholic fatty liver disease by down-regulation of GPAT-1 and DGAT2 expression and inhibition of PKC membranous translocation. Clin. Exp. Pharm. Physiol. 2019, 46, 545–555, doi:10.1111/1440-1681.13074.
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: A systematic review. Child. Obes. 2018, 14, 501–509, doi:10.1089/chi.2018.0040.
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297, doi:10.1038/s41575-020-0269-9.
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464, doi:10.1002/hep.28572.
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764, doi:10.1038/S42255-019-0095-Y.
- Burgermeister, E.; Schnoebelen, A.; Flament, A.; Benz, J.; Stihle, M.; Gsell, B.; Rufer, A.; Ruf, A.; Kuhn, B.; Märki, H.P.; et al. A novel partial agonist of Peroxisome proliferator-activated receptor-γ (PPARγ) recruits PPARγ-coactivator-1α, prevents triglyceride accumulation, and potentiates insulin signaling in vitro. Mol. Endocrinol. 2006, 20, 809–830, doi:10.1210/ME.2005-0171.
- Biondo, L.A.; Junior, E.A.L.; Souza, C.O.; Cruz, M.M.; Cunha, R.D.C.; Alonso-Vale, M.I.; Oyama, L.M.; Nascimento, C.M.O.; Pimentel, G.D.; Santos, R.V.T. dos; et al. Impact of doxorubicin treatment on the physiological functions of white adipose tissue. PLoS ONE 2016, 11, e0151548, doi:10.1371/JOURNAL.PONE.0151548.
- Deng, X.; Pan, X.; Cheng, C.; Liu, B.; Zhang, H.; Zhang, Y.; Xu, K. Regulation of SREBP-2 intracellular trafficking improves impaired autophagic flux and alleviates endoplasmic reticulum stress in NAFLD. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 337–350, doi:10.1016/j.bbalip.2016.12.007.
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061, doi:10.3390/IJMS21062061.
- Guzmán, C.; Benet, M.; Pisonero-Vaquero, S.; Moya, M.; García-Mediavilla, M.; Martínez-Chantar, M.; González-Gallego, J.; Castell, J.; Sánchez-Campos, S.; Jover, R. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARα; and repressed by C/EBPα: Implications in FABP1 down-regulation in nonalcoholic fatty liver disease. Biochim. Biophys. Acta 2013, 1831, 803–818, doi:10.1016/J.BBALIP.2012.12.014.
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–9, doi:10.4291/wjgp.v6.i4.110.
- Jian, C.; Luukkonen, P.; Sädevirta, S.; Yki-Järvinen, H.; Salonen, A. Impact of short-term overfeeding of saturated or unsaturated fat or sugars on the gut microbiota in relation to liver fat in obese and overweight adults. Clin. Nutr. 2021, 40, 207–216, doi:10.1016/j.clnu.2020.05.008.
- Qiao, Y.; Sun, J.; Xie, Z.; Shi, Y.; Le, G. Propensity to high-fat diet-induced obesity in mice is associated with the indigenous opportunistic bacteria on the interior of Peyer’s patches. J. Clin. Biochem. Nutr. 2014, 55, 120–128, doi:10.3164/jcbn.14-38.
- Wang, Y.; Fei, Y.; Liu, L.; Xiao, Y.; Pang, Y.; Kang, J.; Wang, Z. Polygonatum odoratum polysaccharides modulate gut microbiota and mitigate experimentally induced obesity in rats. Int. J. Mol. Sci. 2018, 19, 3578, doi:10.3390/ijms19113587.
- Chen, X.; Sun, H.; Jiang, F.; Shen, Y.; Li, X.; Hu, X.; Shen, X.; Wei, P. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ 2020, 8, e8317, doi:10.7717/peerj.8317.
- Monga Kravetz, A.; Testerman, T.; Galuppo, B.; Graf, J.; Pierpont, B.; Siebel, S.; Feinn, R.; Santoro, N. Effect of gut microbiota and PNPLA3 rs738409 variant on nonalcoholic fatty liver disease (NAFLD) in obese youth. J. Clin. Endocrinol. Metab. 2020, 105, e3575–e3585, doi:10.1210/clinem/dgaa382.
- Haeusler, R.A.; Camastra, S.; Nannipieri, M.; Astiarraga, B.; Castro-Perez, J.; Xie, D.; Wang, L.; Chakravarthy, M.; Ferrannini, E. Increased bile acid synthesis and impaired bile acid transport in human obesity. J. Clin. Endocrinol. Metab. 2016, 101, 1935–1944, doi:10.1210/jc.2015-2583.

