Fermented dairy products are the good source of different species of live lactic acid bacteria (LAB), which are beneficial microbes well characterized for their health-promoting potential. Traditionally, dietary intake of fermented dairy foods has been related to different health-promoting benefits including antimicrobial activity and modulation of the immune system, among others. In recent years, emerging evidence suggests a contribution of dairy LAB in the prophylaxis and therapy of non-communicable diseases. Live bacterial cells or their metabolites can directly impact physiological responses and/or act as signalling molecules mediating more complex communications. This rentryview provides up-to-date knowledge on the interactions between LAB isolated from dairy products (dairy LAB) and human health by discussing the concept of the food–gut-health axis. In particular, some bioactivities and probiotic potentials of dairy LAB have been provided on their involvement in the gut–brain axis and non-communicable diseases mainly focusing on their potential in the treatment of obesity, cardiovascular diseases, diabetes mellitus, inflammatory bowel diseases, and cancer.
- lactic acid bacteria
- dairy food products
- gut microbiota
1. Introduction
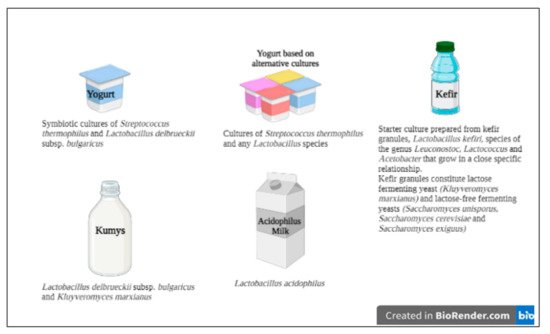
2. Definitions and Some Characteristics of LABs
| LAB | Family | Genus | Gram −/+ |
Growth Conditions | Type of Lactic Acid |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy Products | Isolated Probiotic Strains | Their Bioactivities and Stability Issues | Reference(s) | Heat-Stable (45 °C) |
Salt-Tolerant (18% NaCl) | Acid-Resistant (pH 4.4) |
|||||
| Dairy | |||||||||||
| Kalarei, a traditional fermented cheese product | Pediococcus acidilactici | ||||||||||
| SMVDUDB2 | * An 80% survival rate at low pH (2.0 and 3.0) and high bile salt concentration (0.3 and 0.5%) * High hydrophobicity affinity (33.3%) with ethyl acetate * Autoaggregation (77.68 ± 0.68%) and coaggregation (73.57 ± 0.47%) with Staphylococcus aureus (MTCC 3160) * Antibacterial activity against Bacillus subtilis (MTCC 121), Mycobacterium smegmatis (MTCC 994), Staphylococcus aureus (MTCC 3160), Proteus vulgaris (MTCC 426), Escherichia coli (MTCC 1652), and Lactocaseibacillus rhamnosus (MTCC 1408) |
[37] | Lactobacillaceae | ||||||||
| Ezine cheese (a Turkish cheese) | Lactobacillus | Enterococcus lactis PMD74 | * The strain showed autoaggregative (41%) and coaggregative properties along with high viability at acidic pH (3.0) and in the presence of pepsin, pancreatin, and bile salts (0.3% and 0.5%). * The strain PMD74 inhibited the growth of a number of Gram-positive bacteria (Listeria monocytogenes, Lactobacillus sake, Staphylococcus aureus, and Enterococcus faecalis | + | Changeable | - | Changeable | D, L, DL | |||
| ). | [ | 36 | ] | ||||||||
| Tulum cheese (a Turkish cheese) | Pediococcus | Seven Limosilactobacillus fermentum strains | * | + | Limosilactobacillus fermentum LP3 and LP4 were able to tolerate acidic pH (2.5) and 1% bile salt. * Although all strains had similar enzymatic activity and antibiotic resistance patterns, the highest antagonistic effect belonged to LP3, LP4, and LP6 and the highest cholesterol assimilation belonged to LP3 and LP4, respectively. |
[ | Changeable | - | + | 39] | L, DL |
| Streptococcaceae | |||||||||||
| Probiotic yogurt | Streptococcus | Lactobacillus acidophilus, Bifidobacterium bifidum, Lactiplantibacillus plantarum, Lacticaseibacillus casei | + | Changeable | - | - | L | ||||
| * A combination of | Lactobacillus acidophilus | and Bifidobacterium bifidum survived at pH 1.5 during an incubation period of 1.5 h and also showed good survivability at 0.3% bile salt concentration. * At pH 2.0, 3.0, and 4.0, the survivability rate for Lactobacillus acidophilus and Bifidobacterium bifidum was 54, 66, and 64%, respectively. |
[40] | Lactococcus | + | - | - | Changeable | L | ||
| Yogurt | Streptococcus thermophilus BGKMJ1-36 and Lactobacillus bulgaricus BGVLJ1-21 |
* Both strains grew at 37 and 45 °C in GM17 broth, while they did not grow in GM17 broth with 2% NaCl. * Both strains showed antimicrobial activity toward Listeria monocytogenes, while the BGKMJ1-36 strain produced EPS. * The colonies of BGKMJ1-36 and BGVLJ1-21 strains that successfully survived transit of the yogurt via simulated gastrointestinal tract conditions have been examined for adhesion to intestinal epithelial Caco-2 cells. |
[41] | Propionibacteriaceae | |||||||
| Iranian traditional yogurts | Propionibacterium | 12 LAB isolates from two genera (Pediococcus; | + | 6 P. acidilacticii isolates and Lactobacillus; 2 Lactiplantibacillus plantarum | - | - | - | , 2 Levilactobacillus brevis | |||
| , 1 | Limosilactobacillus fermentum and 1 Lactobacillus kefiri isolates). | * Limosilactobacillus fermentum 27 had the highest acid tolerance, while Levilactobacillus brevis 25 had the highest bile salt tolerance. * Pediococcus acidilactici 23 showed a lower acid tolerance as well as Levilactobacillus Brevis 86 exhibited a lower bile salt tolerance than others. |
[35] | Enterococcaceae | Enterococcus | + | + | - | + | L | |
| Local dairy (cow milk, buffalo milk, cheese, and yogurt) | Lactobacillus alimentarius, Lactobacillus sake, and Lactobacillus collinoides | * The Lactobacillus strains inhibited pathogens’ growth. * All three isolates showed moderate activity apart from Lactobacillus collinoides and Lactobacillus alimentarius, which had relatively strong activity against Pseudomonas aeruginosa and Bacillus subtilis. |
[42] | Leuconostocaecae | Leuconostoc | + | - | - | Changeable | D | |
| 30 dairy samples (household milk and curd) | 12 Lactobacillus isolates (LBS 1-LBS 12) | * Eight isolates (LBS 1-6, 8 and 11) were bile resistant (survival >50% at 0.3% bile salt w/v) and five isolates (LBS 1, 2, 5, 6 and 11) were resistant at acidic pH (survival >50% at pH 3). * All isolates inhibited the growth of Staphylococcus aureus. * LBS 2 also inhibited the growth of Escherichia coli and Salmonella typhimurium. * Isolate LBS 2 was resistant to five antibiotics as well as Lactocaseibacillus rhamnosus LBS2 successfully adhered to rat epithelial cells in in vitro conditions. |
Nondairy | ||||||||
| Aerococcaceae | Aerococcus | + | - | - | - | L | |||||
| Carnobacteriaceae | Carnobacterium | + | - | - | NA | L | |||||
| Enterococcaceae | Tetragenococcus | + | - | + | Changeable | L | |||||
| Enterococcaceae | Vagococcus | + | - | - | NA | L | |||||
| Fructobacillus | + | NA | - | NA | D | ||||||
| Leuconostocaecae | Oenococcus | + | - | - | Changeable | D | |||||
| Weissella | + | - | - | Changeable | D, L | ||||||
NA: Not available, D: Dextrorotary; optical rotation to the right (+), L: Levorotary; optical rotation to the left (−).
3. Importance of LABs in Dairy Foods in Terms of Health
| [ | |||
| 43 | |||
| ] | |||
| Traditional Greek dairy products (Feta, Kasseri, Xynotyri, Graviera, Formaela, Galotyri, and Kefalotyri cheeses as well as yogurt and milk) | |||
| 25 LAB strains | |||
| * Only | Streptococcus thermophilus | ACA-DC 26 (Greek yogurt isolate) had antimicrobial activity (against Streptococcus mutans LMG 14558T). * Two Lactiplantibacillus plantarum strains (ACA-DC 2640 and ACA-DC 4039) showed the highest adhesion according to a collagen-based microplate assay and by using HΤ-29 and Caco-2 cells. * Milk cell-free supernatants of Lactiplantibacillus plantarum ACA-DC 2640 and ACA-DC 4039 showed strong angiotensin I-converting enzyme inhibition. * Lactiplantibacillus plantarum ACA-DC 2640, Streptococcus thermophilus ACA-DC 26, and ACA-DC 170 had anti-inflammatory activity. |
[44] |
| Tibetan kefir | Lactobacillus kefiranofaciens XL10 | * XL10 survived 3-h incubation at pH 3.5 and exhibited cell surface hydrophobicity of ~79.9% and autoaggregation of ~27.8%. * XL10 successfully adhered to the mucous tissue and colonized the ileum of the mice. * XL10 modulated gut microbiota by increasing the Bifidobacteriaceae family and decreasing in Proteobacteria phyla. |
[45] |
| Mongolian fermented koumiss | Lactobacillus helveticus NS8 | * Although NS8 exhibited a moderate survival ability in the gastrointestinal tract environment in vitro, an excellent adhesion ability to human intestinal cells and significant autoaggregation and cell-surface hydrophobicity were reported. * NS8 was able to decline the proinflammatory effects of lipopolysaccharide by inducing higher levels of IL-10. |
[38] |
References
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059.
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Zoumpopoulou, G.; Pot, B.; Tsakalidou, E.; Papadimitriou, K. Dairy probiotics: Beyond the role of promoting gut and immune health. Int. Dairy J. 2017, 67, 46–60.
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912.
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352.
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876.
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 647.
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614.
- Eor, J.Y.; Tan, P.L.; Son, Y.J.; Lee, C.S.; Kim, S.H. Milk products fermented by lactobacillus strains modulate the gut–bone axis in an ovariectomised murine model. Int. J. Dairy Technol. 2020, 73, 743–756.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.-M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a healthy gut: Intake of unpasteurised milk is associated with increased lactobacillus abundance in the human gut microbiome. Nutrients 2020, 12, 1468.
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533.
- Guillemard, E.; Tondu, F.; Lacoin, F.; Schrezenmeir, J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010, 103, 58–68.
- Yu, A.O.; Leveau, J.H.J.; Marco, M.L. Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Environ. Microbiol. Rep. 2020, 12, 16–29.
- He, L.; Li, J.; Sun, Z. More critical consideration on enhancing micronutrient bioavailability of phytate rich foods by phytase-producing lactic acid bacteria. Trends Food Sci. Technol. 2020, 102, 37–38.
- Levit, R.; Savoy de Giori, G.; Moreno de LeBlanc, A.; LeBlanc, J.G. Recent update on lactic acid bacteria producing riboflavin and folates: Application for food fortification and treatment of intestinal inflammation. J. Appl. Microbiol. 2021, 130, 1412–1424.
- Ferreira, C.L.L. Prebióticos e Probióticos: Atualização e Prospecção; Editora Rubio: Rio de Janeiro, Brazil, 2012; ISBN 8564956020.
- Börner, R.A.; Kandasamy, V.; Axelsen, A.M.; Nielsen, A.T.; Bosma, E.F. Genome editing of lactic acid bacteria: Opportunities for food, feed, pharma and biotech. FEMS Microbiol. Lett. 2019, 366, fny291.
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83.
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86.
- Mulaw, G.; Sisay Tessema, T.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented ethiopian food products. Int. J. Microbiol. 2019, 2019, 7179514.
- Marcial-Coba, M.S.; Knøchel, S.; Nielsen, D.S. Low-moisture food matrices as probiotic carriers. FEMS Microbiol. Lett. 2019, 366, fnz006.
- Ershidat, O.T.M.; Mazahreh, A.S. Probiotics bacteria in fermented dairy products. Pakistan J. Nutr. 2009, 8, 1107–1113.
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry—Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982.
- Oh, S. Probiotics in Dairy Products. In Beneficial Microorganisms in Food and Nutraceuticals; Liong, M.-T., Ed.; Microbiology Monographs; Springer International Publishing: Cham, Switzerland, 2015; Volume 27, pp. 203–219. ISBN 978-3-319-23176-1.
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, A.S.M.R.U.; Jahid, I.K.; Rubayet Ul Alam, A.S.M.; Jahid, I.K. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237.
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic viability in yoghurt: A review of influential factors. Int. Dairy J. 2020, 109, 104793.
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249.
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 502.
- Azaïs-Braesco, V.; Bresson, J.L.; Guarner, F.; Corthier, G. Not all lactic acid bacteria are probiotics, …but some are. Br. J. Nutr. 2010, 103, 1079–1081.
- Davoren, M.J.; Liu, J.; Castellanos, J.; Rodríguez-Malavé, N.I.; Schiestl, R.H. A novel probiotic, lactobacillus johnsonii 456, resists acid and can persist in the human gut beyond the initial ingestion period. Gut Microbes 2019, 10, 458–480.
- Milk and Milk Products. Joint FAO/WHO Codex Alimentarius Commission, 2nd ed.; Food & Agriculture Organization: Rome, Italy, 2011.
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 12.
- Sharifi Yazdi, M.K.; Davoodabadi, A.; Khesht Zarin, H.R.; Tajabadi Ebrahimi, M.; Soltan Dallal, M.M. Characterisation and probiotic potential of lactic acid bacteria isolated from Iranian traditional yogurts. Ital. J. Anim. Sci. 2017, 16, 185–188.
- Uymaz Tezel, B. Preliminary in vitro evaluation of the probiotic potential of the bacteriocinogenic strain Enterococcus lactis PMD74 isolated from ezine cheese. J. Food Qual. 2019, 2019, 4693513.
- Bhagat, D.; Raina, N.; Kumar, A.; Katoch, M.; Khajuria, Y.; Slathia, P.S.; Sharma, P. Probiotic properties of a phytase producing pediococcus acidilactici strain SMVDUDB2 isolated from traditional fermented cheese product, kalarei. Sci. Rep. 2020, 10, 1926.
- Rong, J.; Zheng, H.; Liu, M.; Hu, X.; Wang, T.; Zhang, X.; Jin, F.; Wang, L. Probiotic and anti-inflammatory attributes of an isolate Lactobacillus helveticus NS8 from Mongolian fermented koumiss. BMC Microbiol. 2015, 15, 1–11.
- Tulumoğlu, Ş.; Kaya, H.İ.; Şimşek, Ö. Probiotic characteristics of Lactobacillus fermentum strains isolated from tulum cheese. Anaerobe 2014, 30, 120–125.
- Soni, R.; Jain, N.K.; Shah, V.; Soni, J.; Suthar, D.; Gohel, P. Development of probiotic yogurt: Effect of strain combination on nutritional, rheological, organoleptic and probiotic properties. J. Food Sci. Technol. 2020, 57, 2038–2050.
- Popović, N.; Brdarić, E.; Đokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić-Vidojević, A. Yogurt produced by novel natural starter cultures improves gut epithelial barrier in vitro. Microorganisms 2020, 8, 1586.
- Karami, S.; Roayaei, M.; Hamzavi, H.; Bahmani, M.; Hassanzad-Azar, H.; Leila, M.; Rafieian-Kopaei, M. Isolation and identification of probiotic Lactobacillus from local dairy and evaluating their antagonistic effect on pathogens. Int. J. Pharm. Investig. 2017, 7, 137.
- Kumar, A.; Kumar, D. Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Anaerobe 2015, 33, 117–123.
- Zoumpopoulou, G.; Tzouvanou, A.; Mavrogonatou, E.; Alexandraki, V.; Georgalaki, M.; Anastasiou, R.; Papadelli, M.; Manolopoulou, E.; Kazou, M.; Kletsas, D.; et al. Probiotic features of lactic acid bacteria isolated from a diverse pool of traditional greek dairy products regarding specific strain-host interactions. Probiotics Antimicrob. Proteins 2018, 10, 313–322.
- Xing, Z.; Tang, W.; Geng, W.; Zheng, Y.; Wang, Y. In vitro and in vivo evaluation of the probiotic attributes of Lactobacillus kefiranofaciens XL10 isolated from Tibetan kefir grain. Appl. Microbiol. Biotechnol. 2017, 101, 2467–2477.
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the gut microbiota a link between obesity and central nervous system disorders? Curr. Obes. Rep. 2016, 5, 51–64.
- Appleton, J. The gut-brain axis: Influence of microbiota on mood and mental health. Integr. Med. 2018, 17, 28–32.
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489.
- Karakan, T.; Ozkul, C.; Küpeli Akkol, E.; Bilici, S.; Sobarzo-Sánchez, E.; Capasso, R. Gut-brain-microbiota axis: Antibiotics and functional gastrointestinal disorders. Nutrients 2021, 13, 389.
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Liver Physiol. 2012, 303, G1288–G1295.
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E. V Evolution of cell–cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299.
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255.
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013.
- Sobko, T.; Huang, L.; Midtvedt, T.; Norin, E.; Gustafsson, L.E.; Norman, M.; Jansson, E.Å.; Lundberg, J.O. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic. Biol. Med. 2006, 41, 985–991.
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The food-gut human axis: The effects of diet on gut microbiota and metabolome. Curr. Med. Chem. 2019, 26, 3567–3583.
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Kim, D.S.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 2014, 10, 465–474.
- Marcos, A.; Wärnberg, J.; Nova, E.; Gómez, S.; Alvarez, A.; Alvarez, R.; Mateos, J.A.; Cobo, J.M. The effect of milk fermented by yogurt cultures plus Lactobacillus casei DN-114001 on the immune response of subjects under academic examination stress. Eur. J. Nutr. 2004, 43, 381–389.
