1. Surgical Treatment for Oligometastatic Non-Small Cell Lung Cancer
For LAT of oligometastatic NSCLC, surgical resection has traditionally been the main treatment modality, with more than 50% of all patients receiving surgical treatment in early systematic reviews
[1][24]. Berzenji et al. recently summarized the two most common treatment approaches: The first approach (
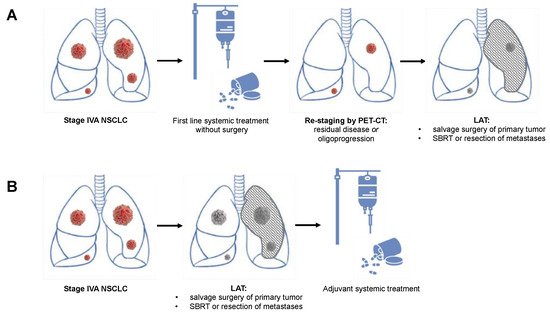
Figure 1B) includes an initial aggressive resection of the primary tumor, followed by the resection or SBRT of metastatic lesions. Systemic treatment (preferably targeted treatment in NSCLC with targetable oncogenic drivers or immunotherapy in NSCLC without targetable oncogenic drivers but PD-L1 expression >1%) is subsequently used for the control of micrometastatic disease
[2][10]. A second option for addressing oligometastatic NSCLC is a neoadjuvant systemic treatment as described above, followed by a PET-CT-based re-staging and subsequent resection (
Figure 1A). In non-progressive or oligoprogressive disease, the resection of the primary tumor and an aggressive treatment of distant metastases by either resection or SBRT follow thereafter in a so-called “salvage” surgery concept
[2][3][10,25]. Upfront surgery offers the advantage of performing surgery without delay and without the risk of a decline in the functional status after an induction treatment. However, no down-staging is possible and extensive open resections such as pneumonectomies or sleeve resections are often required
[4][26].
Figure 1. Schematic presentation of the two potential multimodality approaches including local aggressive treatment (LAT) of oligometastatic non-small cell lung cancer with contralateral pulmonary metastases. (A) Scheme involving systemic induction treatment, followed by re-staging and LAT with salvage surgery in case of residual disease or oligoprogression. (B) Primary LAT with surgical resection or radiation therapy of the primary tumor and all metastatic lesions, followed by adjuvant systemic treatment. LAT: local aggressive therapy.
In contrast, neoadjuvant treatment is administered with the intent to eradicate nodal and micrometastatic disease and achieve a reduction in tumor volume and burden, which subsequently enables a complete resection of the remaining tumor (
Figure 2)
[4][26]. In addition, neoadjuvant systemic treatment is more likely to provide access for both surgical and systemic treatment modalities to a larger number of patients, while a substantial number of patients may not be able to complete adjuvant treatment if an extensive surgery was performed upfront
[4][26]. Finally, neoadjuvant systemic treatment allows assessing the treatment response and treatment-induced changes in tumor biology on histopathological and molecular levels
[4][26]. This information may provide additional guidance to decide on the further treatment steps. What needs to be considered, however, is that surgery after neoadjuvant treatment may be more challenging than upfront surgery. Especially after combination regimens with chemotherapy and immunotherapy, increased vascular fragility and interstitial exudation, compacted or calcified hilar or mediastinal lymph node stations, and fibrotic changes render surgery in these patients more difficult
[5][27]. However, despite these challenges, even extensive resections in locally advanced stages and after induction with immunotherapy can be safely performed with 90-day mortality rates between 0% and 3%
[6][7][8][28,29,30]. The ideal timing of LAT within a multimodality treatment approach is, thus, highly debated and several ongoing clinical trials are currently evaluating different schemes of LAT in combination with targeted therapy, immunotherapy, and/or chemotherapy (
Table 1).
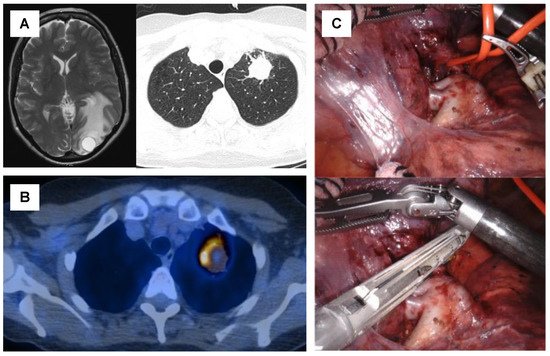
Figure 2. Case of a 57-year-old patient with oligometastatic lung squamous cell carcinoma with a single synchronous brain metastasis and no sign of mediastinal nodal involvement (A). The patient underwent surgical resection of the brain metastasis, followed by six cycles of platin-based chemotherapy. Re-staging by PET-CT showed a stable disease of the primary tumor and no signs of additional metastases (B). Subsequently, a robotic-assisted thoracoscopic (RATS) upper left lobectomy was performed to complete the local aggressive therapy (LAT) (C).
Table 1. Ongoing clinical trials for local aggressive therapy including surgery in oligometastatic non-small cell lung cancer. LAT: local aggressive treatment. OPD: oligoprogressive disease. OS: overall survival. PFS: progression-free survival. SBRT: stereotactic body radiation therapy.
| Study Abbreviation |
ClinicalTrails.gov
Identifier |
Phase |
Setting |
Type of Systemic Treatment |
Type of LAT |
Timing of LAT |
n |
No. of Metastases |
Primary
End Points |
Planned Completion |
| 14-18 CHESS |
NCT03965468 |
II |
Synchronous oligometastatic NSCLC |
Durvalumab, Carboplatin, Paclitaxel |
Primary: Surgery or radical radiotherapyMetastases: SBRT |
Neoadjuvant systemic treatment |
47 |
Max. 3 |
PFS |
12/2021 |
| OMEGA |
NCT03827577 |
III |
Oligometastatic NSCLC |
Standard medical therapy |
Surgery, Radiotherapy, RFA |
Neoadjuvant systemic treatment or primary LAT |
195 |
Max. 3 |
OS |
09/2022 |
| n/a |
NCT02759835 |
II |
EGFR-mutated OPD NSCLC |
Osimertinib |
Surgery, SBRT, radiofrequency ablation |
LAT after oligoprogression under first-lineOsimertinib |
37 |
n/a |
PFS |
09/2022 |
| n/a |
NCT02316002 |
II |
Oligometastatic NSCLC |
Adjuvant Pembrolizumab |
Completed first-line treatment (surgery, SBRT, radiotherapy, chemotherapy) |
Any first-line treatment followed by adjuvant pembrolizumab |
51 |
n/a |
PFS |
09/2022 |
| LONESTAR |
NCT03391869 |
III |
Stage IV NSCLC (incl. OMD subgroup) |
Nivolumab and ipilimumab |
Surgery, radiotherapy |
Combined neoadjuvant and adjuvant immunotherapy |
270 |
n/a |
OS |
12/2022 |
| NORTHSTAR |
NCT03410043 |
II |
EGFR-mutatedStage IIIB or IV NSCLC (incl. OMD subgroup) |
Osimertinib |
Surgery, radiotherapy |
Combined neoadjuvant and adjuvant Osimertinib |
143 |
n/a |
PFS |
01/2023 |
| LAT-FLOSI |
NCT04216121 |
IIb |
EGFR-mutated OPD NSCLC |
Osimertinib |
Surgery, SBRT |
LAT after oligoprogression under first-lineOsimertinib |
39 |
Max. 3 |
PFS |
08/2023 |
Several retrospective cohort studies are reporting promising outcomes of a salvage surgery approach with median OS ranging between 9–75.6 months
[9][10][11][31,32,33], 2–5-year survival rates of 20–75%
[9][12][13][14][15][31,34,35,36,37], and an increased PFS ranging from 5.9 to 43.6 months
[9][10][11][31,32,33]. While many of the patients included in these cohorts were treated with conventional chemotherapy, a further increase in OS and PFS is to be expected in patients who underwent neoadjuvant targeted therapy or immunotherapy. A recent study by Ohtaki et al. supports this assumption: In a retrospective cohort of 36 patients who underwent salvage surgery after EGFR- or ALK-TKI treatment, a 3-year OS and PFS of 75.1% and 22.2% were found
[16][38]. A recent retrospective study by Jones et al. additionally supports the concept of a neoadjuvant induction in stage oligometastatic NSCLC by showing that patients who received neoadjuvant therapy had a significantly improved 5-year OS of 40% when compared to the cohort of patients who had received primary surgery (20% 5-year OS)
[17][39]. However, when compared to neoadjuvant chemotherapy followed by local radiotherapy, primary surgery followed by adjuvant chemotherapy still appears to offer an increased median OS (48 months versus 18 months)
[18][40].
The use of pleurectomy and decortication for malignant pleural effusion or disseminated pleural metastases without extrathoracic disease has only been investigated in small sample sizes
[19][41]. Currently, there is no evidence from larger studies and clinical trials to provide a recommendation for LAT in patients with malignant pleural effusion or disseminated pleural metastases
[19][41].
2. Radiation Therapy for Oligometastatic Non-Small Cell Lung Cancer
Data on the use of radiation therapy for oligometastatic NSCLC are currently limited, but the majority of the contemporary data supports the use of consolidative SBRT in patients with stable disease or partial response to first-line systemic treatment or in patients with oligoprogression during systemic therapy
[20][42]. Especially in the era of immunotherapy, the combination of SBRT and immune checkpoint inhibitors has been shown to modulate the tumor microenvironment and increase the trigger for a systemic anti-cancer response
[20][21][42,43]. Most current studies and guidelines recommend an upfront systemic therapy, followed by LAT using SBRT with or without surgery. The American Radium Society currently recommends a cutoff of three metastatic sites or fewer to receive consolidative SBRT
[20][42]. In patients with four to five metastatic lesions, SBRT should be considered on a case-by-case basis. However, the current consensus guidelines are based on smaller phase II trials, while results from larger phase III trials are pending
[20][42]. An enrollment in an ongoing phase 3 trial is, therefore, encouraged when SBRT is planned in patients with oligometastatic NSCLC
[20][42].