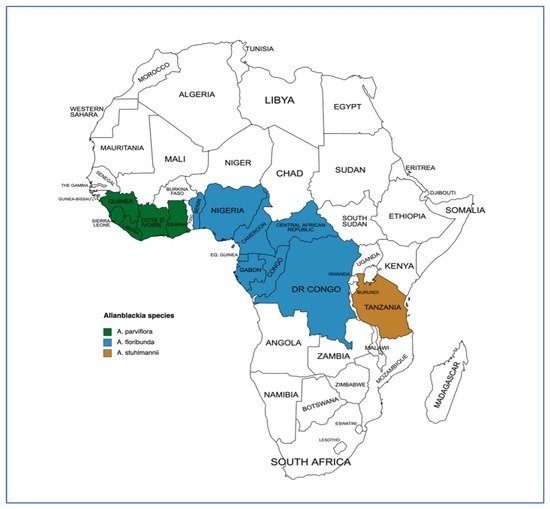
The importance of
A. parviflora includes the provision of timber, shade, and medicine, while the production of oil from seeds is considered the most important economic use
[16][31][40][19,35,44]. In Liberia, the wood (usually called lacewood) is traditionally used in house construction for walls, doors and window frames
[24][29]. It is a potential candidate species for use in agroforestry systems due to its attributes as an alternative income source as well as its ability to provide shade to other crops such as cocoa
[41][45]. The pounded bark can be rubbed on the body to relieve pain. In Côte d’Ivoire, a decoction of the fruit pulp is used to treat elephantiasis of the scrotum
[24][29]. The seeds are usually eaten by children as a high-energy snack
[42][43][46,47]. Some wild animals such as the giant rat, squirrels, and brush-tailed porcupine also depend on
Allanblackia seeds for their survival
[14][17]. The kernel consists of about 67%–73% solid white fat when dried
[14][15][17,18]. The pressed crude oil from the dry seeds is approximately one-third of the seed dry weight
[44][45][48,49], and has traditionally been used for cooking and soap making
[16][46][47][19,50,51]. It has also been reported that the seedcake can be used as a protein-rich animal feed after the seeds are ground and pressed to extract the oil
[43][47]. A recent study also suggests the potential of
A. parviflora seed oil as an alternative for biodiesel production
[17][20]. The seeds, when harvested in large quantities for export, could serve as an important income generation source for producing countries. Market value chains for
Allanblackia seeds collected from wild stands and/or remnants from farmlands have been developed by Unilever (which provides a guaranteed price for harvested seeds) and other commercial parties in Ghana, Nigeria, Cameroon, and Tanzania
[18][41][44][21,45,48]. Aside from the increase in livelihood opportunities for farmers participating in this rural-based enterprise, it also contributes to biodiversity conservation in these landscapes. Unilever discovered new uses of
Allanblackia seed oil at an industrial level to produce cosmetic products and margarine
[18][21], thus raising the international demand to a commercial scale. The
Allanblackia seed oil is superior to other alternative oils such as palm oil due to its moderately high melting point
[14][15][17,18]. Oppong
[48][52] pointed out that Unilever needs 2000 t of
A. parviflora seeds from Ghana; however, only 110 t (just 5.5%) on average are supplied annually
[49][53], emphasizing its importance and need for large-scale planting. The
Allanblackia seed oil has received an endorsement from the European Union (EU) Novel Food Regulations that approves its safe usage in food products
[50][54].
6. Chemical Composition of A. parviflora Seeds
A study conducted on the fatty acid composition of
A. parviflora seeds revealed that their composition is primarily of stearic (51.6%) and oleic acid (43.9%), with minor quantities of myristic (1.8%), palmitic (2.5%), and eicosanoic acid (0.2%). The main triglyceride constituents were identified as 2-oleostearin (60.1%), 1-stearo-diolein (26.9%) and 2-oleopalmitostearin (6.9%)
[51][55]. Stearic acid percentages ranging from 44% to 66% and oleic acid ranging from 25% to 48% per tree sample were documented for
A. floribunda [52][56].
A. floribunda seeds have also been reported to contain a lot of edible oil (67.6%) which is rich in stearic acid
[53][57], and these values are similar to those observed for
A. parviflora seeds. The values confirm the high fat content in the seeds of
Allanblackia species. Adubofuor et al.
[47][51] researched the seeds of
A. parviflora grown in Ghana, from which oil was extracted by either the use of a screw press or Soxhlet extraction (petroleum ether), revealing an average of 68% oil. The gas chromatography method was employed to assess the fatty acid composition of the seed oil as 2.9% palmitic acid, 52.3% stearic acid, and 44.8% oleic acid. The key minerals found in the seeds were potassium (8.41 mg/kg) and phosphorus (8.34 mg/kg). Nutritional analyses showed that the seeds contained 4.3% protein, 2.0% ash, 5.7% crude fibre and 17.1% carbohydrates, with the moisture content being 3.4%. Additionally, Sefah et al.
[54][58] confirmed the moisture content of
A. parviflora seeds to be 3.2%.
7. Morphological and Genetic Diversity
Genetic diversity analysis is very essential in any tree improvement programme for the identification of quality genotypes and proper clonal deployment. Both morphological
[4] and molecular
[55][56][24,25] differences among
Allanblackia tree species show significant genetic diversity within the species. Peprah et al.
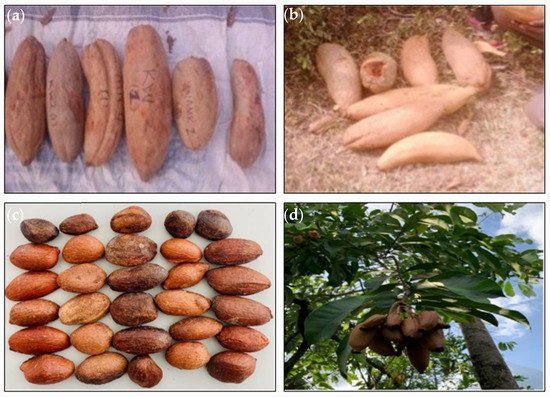
[4], in a study on variation in fruits and seed morphology of 109
A. parviflora trees growing in different parts of Ghana, reported no differences in fruit yield, fruit shape and seed health among the ecological zones. However, significant differences in fruit shape and size were observed among the individual trees sampled (
Figure 4). According to Leakey et al.
[57][66], variation in fruit parameters at the level of the provenance indicates that genetic variation exists within the species since the environment is similar. The results suggest a high genetic improvement potential through individual selection
[4]. In the interim, the adoption of fruit size and seed yield as targeted selection criteria is assumed to be a valid approach for collection and has been used in sampling. The seedlings and grafts raised from these selected trees are disseminated to farmers for farmland cultivation. Moreover, the vegetative propagules (grafts, seedlings, and cuttings) from these superior trees are employed for the setting up of mother blocks, i.e., constructed plots consisting of grafts, seedlings, and cuttings for advanced (vegetative) propagation, and the creation of gene banks for the purposes of conservation
[29][58][33,67]. In Ghana, two mother blocks have been constructed with 20 superior clones. Moreover, a 3 ha gene bank established with seedlings from 120 mother trees has been constructed in addition to the clonal stocks in the mother blocks
[30][34].
Other species of the
Allanblackia genus have received some level of research attention in terms of comparing morphological data to the genetics of a given provenance which is vital for developing sound and effective domestication strategies. For instance, morphological and genetic diversity assessment using genetic markers have been reported for
A. floribunda in Cameroon
[55][56][24,25] and
A. stuhlmannii in Tanzania
[56][59][25,68]. However, there is very limited knowledge on studies focusing on the genetic diversity of
A. parviflora in West Africa using molecular markers even though some work has been done on the morphological aspects in Ghana
[4][14][54][4,17,58]. Genetic markers are employed, for instance, for the estimation of differences between natural and domesticated plant populations, gene flow, fingerprinting, genetic structure, and hybridization, and are therefore essential in breeding programmes and development of new varieties
[60][61][69,70]. Modern molecular markers such as DNA chips and sequencing-based DNA markers (for example, single nucleotide polymorphisms—SNPs) are used for the assessment of genetic diversity and are based on phenotypic differences that are controlled genetically
[62][71]. However, the use of genetic markers, such as SNPs, for genetic diversity studies of
Allanblackia in the West African region where the species’ occurrence has not been adequately explored. Several factors usually make SNPs the preferred choice of markers because they are platform-independent, reproducible across laboratories, and the subsequent databases can be shared worldwide. Moreover, due to the high frequency of SNPs, ease of design from transcriptome or genome assemblies, and the availability of high throughput SNP assay platforms, SNP genotype data are easy to collect in large amounts
[63][72].
8. Conclusions
A. parviflora is one of the priority fruit tree species identified for improvement and domestication in Sub-Saharan Africa. It has been targeted for improvement for the production of oil from the seeds, for benefits not only to the local populations but also the economies of producing countries. Despite the great potential of this species, there are still significant knowledge gaps that require urgent research attention.
This rentryview shows that the most important economic use of A. parviflora is the production of edible oil from the seeds. This might justify why ICRAF and its partners already started with the first domestication steps by selecting superior individuals/populations in Ghana, Cameroon, Nigeria, and Tanzania, while Unilever remains a major buyer of the Allanblackia seed oil. However, scientific information on the seeds’ phytochemical composition and variability is scarce. Additionally, only a little or no information is available on the diversity and management of the species in the regions where it is naturally found. More specifically, basic information on genetic diversity, silvicultural management, productivity, methods of propagation and cultivation are not well documented. The development of morphological descriptors, the use of modern molecular markers for genetic diversity assessment, and studies on the phytochemistry of seeds are therefore highly recommended. Moreover, qualitative research on the preferences of local populations who produce A. parviflora seeds should be conducted to reveal their preferred traits of interest for development. The selection of superior mother tree populations may begin once these attributes are determined. The next step after identifying trees with superior desirable traits will be the multiplication of best individuals. Extensive studies on vegetative propagation and propagule regeneration of the species are urgently needed, as the currently available information is not ample. Seedlings from generative propagation are currently the easiest solution for farmers, but there is a limitation to such a method due to the dioecious nature of the species which makes it difficult to identify the sex of the tree at the juvenile stage. While research efforts should be focused on determining the sex of trees at a young age, studies on multiplication by vegetative propagation is crucial, since such a method of propagation assures the conservation of the traits of interest. The researchers conclude that A. parviflora has great potential to ensure food and nutritional security and alleviate poverty among rural populations in West Africa, and therefore could be domesticated to promote widespread planting in agroforestry systems and for the conservation of the genetic resource if the knowledge gaps identified here are fulfilled.
on the diversity and management of the species in the regions where it is naturally found. More specifically, basic information on genetic diversity, silvicultural management, productivity, methods of propagation and cultivation are not well documented. The development of morphological descriptors, the use of modern molecular markers for genetic diversity assessment, and studies on the phytochemistry of seeds are therefore highly recommended. Moreover, qualitative research on the preferences of local populations who produce A. parviflora seeds should be conducted to reveal their preferred traits of interest for development. The selection of superior mother tree populations may begin once these attributes are determined. The next step after identifying trees with superior desirable traits will be the multiplication of best individuals. Extensive studies on vegetative propagation and propagule regeneration of the species are urgently needed, as the currently available information is not ample. Seedlings from generative propagation are currently the easiest solution for farmers, but there is a limitation to such a method due to the dioecious nature of the species which makes it difficult to identify the sex of the tree at the juvenile stage. While research efforts should be focused on determining the sex of trees at a young age, studies on multiplication by vegetative propagation is crucial, since such a method of propagation assures the conservation of the traits of interest. We conclude that A. parviflora has great potential to ensure food and nutritional security and alleviate poverty among rural populations in West Africa, and therefore could be domesticated to promote widespread planting in agroforestry systems and for the conservation of the genetic resource if the knowledge gaps identified in our review are fulfilled.