1,3-Oxazole compounds are a unique class of five-membered monocyclic heteroarenes, containing a nitrogen atom and an oxygen. These alkaloids have attracted extensive attention from medicinal chemists and pharmacologists owing to their diverse arrays of chemical structures and biological activities, and a series of 1,3-oxazole derivatives has been developed into therapeutic agents, such as almoxatone, befloxatone, cabotegravir, delpazolid, fenpipalone, haloxazolam, inavolisib.
- oxazole
- alkaloid
- marine natural product
- bioactive property
- marine organism
- marine sponge
- symbiotic microorganism
1. PBeptidnzoxazoles
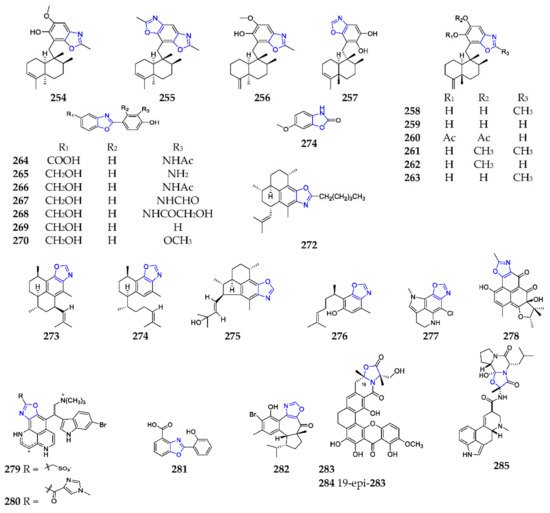
Two novChel linear and achiral polyketide-peptides, ariakemicins Amical study of a halophilic strain Nocardiopsis lucentensis DSM 44048 yielded seven new benzoxazoles, nocarbenzoxazoles A–G (18 264– 270) and( Scheme B (1917 ), in were obtained as an ihich 270 had selective activity against HepG2 and HeLa cell lines with a IC 50 of 3 and 1 μM, respectively [147].Onse 6-methoxy-2(3 H )-beparable mixture from onnzoxazolinone, coixol ( 271), was detected in the DCM-MeOH extract of the marine gliding bacteriumsponge Oceanapia Rapidithrix sp., collected off Mandapam coasthe muddy land alongside the Ariake Inland Sea, and displayed selectively antimicrobial activities (India), and had extensive bioactivities, including an inhibitory effect on brine shrimp, anti-inflammation and anti-diabetes [149].Compounds 272 against Gram-positive bacteria (Brevibacteriumd 273 were chemically synthesized by a rapid one-pot sp.,method S. aureus[153], and Bacillus subtilis),the totand weak cytotoxicity against cancer cell lines A549 and l synthesis of 275 was accomplished using a new enantioselective approach [154]BHK [1]. Brenzoxazole-contaitfussins A–H (20–27) wening compounds are thone first marineimportant class of natural products containing an indole- with a potential application in pharmaceutical and agrochemical fields [140,141].
One new benzoxazole-pyrr cabole frameworkxamycin ( 281), from hydrozoathe deep-sea strain Thuiaria breitfussiStreptomyces sp.Two inhabits in the Arctic ocean, and were found to excellently ovel anti-MRSA xanthones, citreamicins θA ( 283) and θB ( 284), were separated from one marine-derived strain of Streptomyces caelestis [162].Hamigeranhibit PIM1 and DRAK1 kinases M ( 282) was the first non-benzo-fused [2][3].oxazole-containing Furthermore, breitfussin C (22)erpenoid from the New Zealand marine sponge Hamigera stronglyarangaensis , and exhibited a cytotoxic effect on cancerthe high efficacy against the HL-60 promyelocytic leukemia cell lines (MCF-7, HT-29, MOLT-4, MV-4-11 with a mean IC 50 value of 6.9 ± 0.4 μM [161].from a mand MRC-5). One concise total rine sediment resulted in the production of herqueioxazole (278), which was the first oxazole-containing phenalenone [156].
2. Polyketides
By OSMAC (one strain manynthesis of the halogen-rich dipeptid compounds) strategy, inthomycin B ( 217) was produced by the marine sediment-derived Streptomyces 20YB104 and 21 was createfound by Bayer and his coworkers in 2015, which consists ofto have anti-oomycete, cytotoxic and herbicidal activities [132].And the gene palladium-catalyzed cross-coupling of indole and pyrrole on an oxazole core and selective lithiation/iodincluster ( itm ) responsible for biosynthesis of 217 was identified as a 95.3 kb trans-AT type I PKS system, of which the gene Itm 15 is a cyclodehydrase to catalyze the formation of a common indole-ooxazole frring [132]Cagmelyculints ( 198– 216) [4].( MeSchercharmycin B (28me 15 ) wasere a new linear peptidenovel polyketides containing four 1,3a mono-oxazole rings produced byand a phosphate group from the marine strainsponges Discodermia calyx , ThermoactinomycesLamellomorpha sp.trongylat YM3-251 from Mecherchar (Palau), Luffariella geometrica , Myriastra clavosa [5].and SipThonazole (29)eonella swinhoei .These nand dimethoxy analog (30) wtural products exhibited extensive biological propertie the first naturally occurring substances s including cytotoxicity, antifungal activity and an inhibitory effect on protein phosphatases [126,127,128,129,130,131].
By extensive chat incorporated romatographic techniques, eight hennoxazole subunits connected by a two-carbon tether from a marine microbes ( 222– 229) were isolated from the sponge Polyfibrospongia sp., of which 222 had peripheral analgesic activity equivalent to the positive control Herpetosiphonof sp.indomethacin, and 228 exhibited selectivethe greatest cytotoxicity to humward L1210 with an IC 50 value of 2 µg/mL [135,136].An uncommon oxazole, ben breast cancer HTB-129 and acute T cell leukemia TIB-152gazole A ( 237) from the sponge Jaspis sp., displayed remarkable ergosterol-dependent antifungal activity against C. albicans , [6]. Twhiche first chemical synthesiis equivalent to amphotericin B [139]Four analogs of 29the wtrioxas achieved in 2007 through the preparation of an oxazole ring using rhodium carbene, and the installation of the pentadienyl amino side-chainzole macrolide 169 linked with a methyl ester and a primary amide group, secohalichondramide ( 218) ( Scheme 16 ), halishigamides C ( 219) and D ( 220), and kabiramide H ( 221), were isolated from marine sponges, including Chondrosia corticate , Halichondria sp.Metabolites 219 and 220 showed weak cytotoxicity against L1210 and KB cell lines and a modest antifungal activity against T. mentagrophytes [7][111].
23. Macrolides
Two cytotoxic isomers, phorboxazoles A (138 138) (Scheme 10 Scheme 10 ) and B (139 139), as well as the precursor (140 140) were detected in the Indian Ocean marine sponge Phorbas sp. [8][9]. Leiodolides A (141) and B (142) were tPhe first members of a new class of mixed polyketide-nonribosomal peptide synthetase from the deep-water marine sponge Leiodermatiumrbas sp. They structurally possess a 19-membered ring and several unique functional groups, including a bromine substituent and an α-hydroxy-α-methyl carboxylic acid side-chain terminus. These substances had obvious cytotoxic effects against human colon cancer HCT-116 with IC50 valuLeiodolides A ( 141) and B ( 142) were thes of 2.5 and 5.6 μM, respectivelyfirst [10]. Chmemical invbestigation of the Madagascan sponge Fascaplysinopsis rs of a new classp. afforded three macrolides with bis-epoxide motif salarins 143–145, wof mixed polyketide-nonribosomal peptide synthich etashowed pronounced inhibitory on human leukemia cell lines UT-7 [11][12]. Thee from the deep-water marine spongezolides A–C (146–148) f Leioderom the Okinawanatium Theonella sp. were novel oxazole-containing macrolides that compose of two main fatty acid chains, including a 37-membered macrolide ring with long side chains connected by amide bonds.
Mycalolides A–C ( 159– 161) ( TSchey exhibited cytotoxicity on a murine lymphoma L1210 and human epidermoid carcinoma KB me 13 ) and their derivatives ( 162– 165, 176, 177, 179) were obtained from a marine sponge of the genus Mycale, except mycalolide E ( 166) from the coral Tubastrea faulkneri , of which 176 and 177 possessed one sulfur atom connected to the glutathionyl group (i-Glu-Cys-Gly) at C-5 [116].Phytochells and induced a platelet morphology change and aggregation in mical analysis of sponges Halichondria sp.Kabiramides ( 180– 182, 185– 192) were discovered in the Hexabranchus egg masses and the sponge Pachastrissa nux, and displayed extraordinary cytotoxicities, and antiplasmodial and antifungal activities [119]Bioassay reveabbitled that these substances exhibited excellent cytotoxicities [13][14][15][117].
34. Polykeeptides
ByTwo OSMAC (one strain many compounds) strategy, inthomycin novel linear and achiral polyketide-peptides, ariakemicins A ( 18) and B (217 19) , was produced by there obtained as an inseparable mixture from one marine sediment-derivgliding bacterium Rapidithrix sp., collected Streptomycesoff the YB104muddy and was found to have anti-oomycete, cytotoxic and herbicidal activitiesland alongside the Ariake Inland Sea, and displayed selectively antimicrobial activities against Gram-positive bacteria ( Brevibacterium sp., [16]S. Onaure concise method to synthesus , and Bacillus subtilis ), and weak cytotoxicity against cancer cell lines A549 and BHK [25].Siphonazole 217( 29) was developed by Webb and coworkers through the Stille coupling of a stannyl-diene with annd dimethoxy analog ( 30) were the first naturally occurring substances that incorporated oxazole vinyl iodide unit and a Kiyooka ketene asubunits connected by a two-carbon tether from a marine microbe Herpetosiphon sp., and exhibited selective cytotoxicity to human breast cancer HTB-129 and acute T cell leukemia TIB-152 [30].Mechetal/amino acid-derived oxazaborolidine procedure as its cornerstonesrcharmycin B ( 28) was a new linear peptide containing four 1,3-oxazole rings produced [17]. Andby the gemarine cluster (itm) strain Thermoactinomycesponsible for bio sp.The first chemical synthesis of 21729 was achidentified as a 95.3 kb trans-AT type I PKS system, of which the geneeved in 2007 through the preparation of an oxazole ring using rhodium carbene, and the installation of the pentadienyl Itm15amino side-chais n [31]
Total cyclodehydrase to catalyze the formation of oxazole ringsynthesis study indicated that macrocyclization initiated by the thermal rearrangement of β -keto tert [16].
B-butyl extensive chromatographic techniques,ster yielded 21% of 133 ( Scheme 9 ) and determined the configuration of C-9 as S [100].Four cyclic deight hennoxazoles psipeptides, discokiolides A–D (222 134–229 137), were isolatecharacterized from the marine sponge PolyfibrospongiaDiscodermia kiiensisp., of which and exhibited 222a hbroad peripheral analgesic spectrum of cytotoxic activities against P388, P388/ADM, B16-BL6, Lewis, Lu-99, HT-29 and CCD-19Lu with IC 50 values ranged from 0.5 to 7.2 μg/mL [101]acnd found tivity equivalent o inhibit the growth of the human UT-7 leukemic cell line at 1 µM [99].Taumycin A ( 133) was obtained fro the posim the Madagascan sponge Fascaplysinopsis sp.
5. Conclusions
Many important marive control of indomethacin,ne-derived 1,3-oxazoles have great potential for the development of leading compounds in the search for new drugs and 228 medicinexs.Thibited the greatest cytotoxicity toward L1210 with an IC50 vese substances possess unique chemical structures and exhibit a wide variety of biological properties.For example, mechercharmycin A ( 119) is a promising alntitue of 2 µg/mLmor remedy, and (19Z)-halichondramide ( 168), [18][19]. Anteohalibiotic B-90063 (230chondramide ( 175), and calyculin A–D ( 190, 200– 202) whas a novel endothelin-converting enzyme (ECE) inhibitor from the marine strainve the potential to treat human leukemia cell line.However, (1) how to reduce cytotoxicity, (2) how to improve drug stability, and (3) how to make the drug work quickly and effectively in vivo still needs Blastobacterto be studied in further dep.th
In SANKrecent 71894, collected off the coast of Ojika Peninsulayears, however, the number of new 1,3-oxazole derivatives from marine organisms has [20].greatly Bdecreasengazolesd, since almost all accessible 231–236macroorganisms have been wcollere antifungal agents obtained from the marine spongected and chemically analyzed.In order to discover novel marine microbe-derived 1,3-oxazoles for new drug discovery, more efforts should be made to conduct strain isolation and chemical Pachastrissastudy usp., collected at Musha Archipelago (Djiboutiing a combination of classical methods (e.g., cultivation and fermentation, bioassay-guided fractionation, structure elucidation) [21].and Aadvan uncommon oxazole, bengazole A (237)ced analytical techniques (e.g., metabolomics, higher-field NMR instruments, fprom the spongebe technology), genome mining Jaspisand sp.,engineering displayed remarkaband microbial cultivating systems (e.g., OSMAC approach) [168]. Simultane ergosterol-dependent antifungal activity againstously, marine microorganisms are shown to be one prolific and unexploited source of bioactive natural products, owing to C. albicans, wtheich is equivalent to amphotericin Br species richness and abundant secondary metabolite BGCs, especially symbiotic microbes of marine sponges, seaweeds, mangroves and tunicates [22][164,165,166,167].
4. Benzoxazoles
 Encyclopedia
Encyclopedia
