Fermented dairy products are the good source of different species of live lactic acid bacteria (LAB), which are beneficial microbes well characterized for their health-promoting potential. Traditionally, dietary intake of fermented dairy foods has been related to different health-promoting benefits including antimicrobial activity and modulation of the immune system, among others. In recent years, emerging evidence suggests a contribution of dairy LAB in the prophylaxis and therapy of non-communicable diseases. Live bacterial cells or their metabolites can directly impact physiological responses and/or act as signalling molecules mediating more complex communications. This rentryview provides up-to-date knowledge on the interactions between LAB isolated from dairy products (dairy LAB) and human health by discussing the concept of the food–gut-health axis. In particular, some bioactivities and probiotic potentials of dairy LAB have been provided on their involvement in the gut–brain axis and non-communicable diseases mainly focusing on their potential in the treatment of obesity, cardiovascular diseases, diabetes mellitus, inflammatory bowel diseases, and cancer.
1. Introduction
Fermented milk and dairy products are the milestones of dietary lifestyle around the world. Beyond their nutritional and organoleptic properties, the health benefits of fermented dairy products have long been known
[1]. In particular, fermented dairy products are a good source of different species of live lactic acid bacteria (LAB), and beneficial microbes are well characterized for their probiotic potential
[2]. Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit on the host
[3]. The ingestion of probiotics harmonises the composition of commensal microorganisms of the intestinal and urogenital environment by competing with pathogens for nutrients and binding sites and by the in situ production of antimicrobial metabolites
[4]. In addition, probiotics contribute to improving the mucosal barrier function by modulating immune responses in the host and controlling symptoms of inflamed gastrointestinal conditions like those observed in inflammatory bowel disease (IBD)
[5]. Less obvious is the contribution that dairy LAB may exert in the prevention and therapeutic of different human diseases including gynaecological, reproductive, metabolic, cardiovascular, osteoporosis, as well as apoptosis control
[6].
The complex enzymatic heritage of LAB strains contributes to the release of several bioactive metabolites either into the dairy matrix or in the gut, thus promoting the fascinating concept of the food–gut-health axis. These metabolites directly affect different physiological processes or act as signalling molecules to surrounding microorganisms. For example, encrypted bioactive peptides mainly produced by dairy LAB from the catabolism of alpha and beta-caseins, albumin, and globulins have been reported as anti-microbials, hypercholesteraemics, opioid and opioid antagonists, angiotensin-converting enzyme inhibitors, anti-thrombotics, immunomodulators, cytomodulators, and anti-oxidants
[7]. There is evidence that the consumption of probiotics-containing dairy products such as yogurt, cultured fermented milk, and kefir has been associated with a range of health benefits including cholesterol metabolism and angiotensin-converting enzyme (ACE) inhibition, antimicrobial activity, tumour suppression, increased speed of wound healing, and the modulation of the immune system
[8][9][8,9]. Recently, dairy products fermented by Lactobacillus strains were reported to modulate the gut-bone axis in a murine model; this is an effect that can be modulated by living Lactobacillus cells as well as dairy products fermented by the same
Lactobacillus
[10]. In the food–gut complex ecosystem, dairy LAB can induce a network of signals mediated by the whole bacteria or their components. The gut–brain–microbiota axis is based on a bilateral communication system through signalling from gut-microbiota to brain and from brain to gut-microbiota by the involvement of neural, endocrine, immune, and metabolic links
[11]. In a preliminary study, Butler and co-authors observed that dietary changes based on the intake of dairy products promote the
Lactobacillus abundance and suggest a link with the psychological status in participants by measuring the predictive neuroactive potential using a gut–brain module approach
[12]. Accordingly, a recent survey indicated that the intake of dairy products such as milk, yogurt, and kefir may modulate the gut microbiota by increasing the
Lactobacillus population
[13], while consumption of a fermented dairy product containing a probiotic
Lactobacillus casei strain reduces the duration of respiratory infections in the elderly
[14]. However, the influence of dairy consumption in conjunction with other factors such as host genetics, age, sex, and lifestyle influences the gut microbiota composition and functionality.
2. Definitions and Some Characteristics of LABs
LAB are a phylogenetically and functionally diverse group of bacteria
[15] and are widely known as microbial food cultures
[16]. By definition, LAB constitute a phylogenetically and functionally diverse taxonomic order of bacteria
[15]. These diverse microorganisms have a common defining characteristic: they produce lactic acid as the end result in the process of fermenting carbohydrates
[17][18][19][17,18,19]. Traditionally, they are closely linked to the fermentation of human foods especially in dairy products
[16]. The general characteristics seen in LAB are summarized in
Table 1.
Table 1. Defined lactic acid bacteria (LAB) and their most common characteristics
[20].
| LAB |
Family |
Genus |
Gram
−/+ |
Growth Conditions |
Type of
Lactic Acid |
| |
|
|
|
Heat-Stable
(45 °C) |
Salt-Tolerant (18% NaCl) |
Acid-Resistant
(pH 4.4) |
|
| Dairy |
|
|
|
|
|
|
|
| |
Lactobacillaceae |
Lactobacillus |
+ |
Specific starter culture(s) of some dairy products (adapted from Codex Alimentarius, 2011).
Some LAB strains isolated from dairy products, their potential bioactivities/probiotic potentials and stability issues are summarized in
Table 2. LAB strains isolated from fermented dairy products are generally viable in low acidic conditions and various bile acid concentrations. However, some LAB have also been reported to show low stability under the same conditions
[35]. In addition, many LAB have been reported to have the superb capability of adhesion to human intestinal cells and important auto-aggregation properties, as well as antimicrobial and immunomodulatory activities
[36][37][38][36,37,38]. Available data support the high potential of LAB in novel functional food production. However, further studies are recommended to select the ideal combination and environment of probiotic strains as well as novel probiotic strains in the development of new probiotic products in the dairy industry.
Table 2.
Some LABs isolated from dairy products, Their Potential Bioactivities & Probiotic Potentials and Stability Issues.
| Dairy Products |
Isolated Probiotic Strains |
Their Bioactivities and Stability Issues |
Reference(s) |
| Kalarei, a traditional fermented cheese product |
Pediococcus acidilactici |
SMVDUDB2 |
* An 80% survival rate at low pH (2.0 and 3.0) and high bile salt concentration (0.3 and 0.5%)
* High hydrophobicity affinity (33.3%) with ethyl acetate
* Autoaggregation (77.68 ± 0.68%) and coaggregation (73.57 ± 0.47%) with | Staphylococcus aureus | (MTCC 3160)
* Antibacterial activity against | Bacillus subtilis | (MTCC 121), | Mycobacterium smegmatis | (MTCC 994), | Staphylococcus aureus | (MTCC 3160), | Proteus vulgaris | (MTCC 426), | Escherichia coli | (MTCC 1652), and | Lactocaseibacillus rhamnosus | (MTCC 1408) |
[37] |
| Ezine cheese (a Turkish cheese) |
Enterococcus lactis | PMD74 |
* The strain showed autoaggregative (41%) and coaggregative properties along with high viability at acidic pH (3.0) and in the presence of pepsin, pancreatin, and bile salts (0.3% and 0.5%).
* The strain PMD74 inhibited the growth of a number of Gram-positive bacteria ( | Listeria monocytogenes, Lactobacillus sake, Staphylococcus aureus | , and | Enterococcus faecalis | ). |
[ | Changeable |
- |
Changeable |
36] | D, L, DL |
| |
|
Pediococcus |
| Tulum cheese (a Turkish cheese) |
Seven | Limosilactobacillus fermentum | + |
strains |
* | Limosilactobacillus fermentum | LP3 and LP4 were able to tolerate acidic pH (2.5) and 1% bile salt.
* Although all strains had similar enzymatic activity and antibiotic resistance patterns, the highest antagonistic effect belonged to LP3, LP4, and LP6 and the highest cholesterol assimilation belonged to LP3 and LP4, respectively. | Changeable |
- |
+ |
L, DL |
| [ | 39 | ] |
|
Streptococcaceae |
Streptococcus |
+ |
Changeable |
- |
- |
L |
| Probiotic yogurt |
Lactobacillus acidophilus, Bifidobacterium bifidum, Lactiplantibacillus plantarum, Lacticaseibacillus casei |
* A combination of | Lactobacillus acidophilus | and | Bifidobacterium bifidum | survived at pH 1.5 during an incubation period of 1.5 h and also showed good survivability at 0.3% bile salt concentration.
* At pH 2.0, 3.0, and 4.0, the survivability rate for | Lactobacillus acidophilus | and | Bifidobacterium bifidum | was 54, 66, and 64%, respectively. |
[40] |
|
|
Lactococcus |
+ |
- |
- |
| Yogurt |
Streptococcus thermophilus | BGKMJ1-36 and | Lactobacillus bulgaricus |
BGVLJ1-21 |
* Both strains grew at 37 and 45 °C in GM17 broth, while they did not grow in GM17 broth with 2% NaCl.
* Both strains showed antimicrobial activity toward | Listeria monocytogenes, | while the BGKMJ1-36 strain produced EPS.
* The colonies of BGKMJ1-36 and BGVLJ1-21 strains that successfully survived transit of the yogurt via simulated gastrointestinal tract conditions have been examined for adhesion to intestinal epithelial Caco-2 cells. |
[ | Changeable |
41] | L |
| |
Propionibacteriaceae |
Propionibacterium |
+ |
- |
- |
- |
|
| Iranian traditional yogurts |
12 LAB isolates from two genera ( | Pediococcus; | 6 | P. acidilacticii | isolates and | Lactobacillus | ; 2 | Lactiplantibacillus plantarum | , 2 | Levilactobacillus brevis | , 1 | Limosilactobacillus fermentum | and 1 | Lactobacillus kefiri | isolates). |
* | Limosilactobacillus fermentum | 27 had the highest acid tolerance, while | Levilactobacillus brevis | 25 had the highest bile salt tolerance.
* | Pediococcus acidilactici | 23 showed a lower acid tolerance as well as | Levilactobacillus Brevis | 86 exhibited a lower bile salt tolerance than others. |
[35] |
|
Enterococcaceae |
Enterococcus |
+ |
+ |
- |
+ |
| Local dairy (cow milk, buffalo milk, cheese, and yogurt) |
Lactobacillus alimentarius, Lactobacillus sake, | and | Lactobacillus collinoides |
* The | L |
| Lactobacillus | strains inhibited pathogens’ growth. |
* All three isolates showed moderate activity apart from | Lactobacillus collinoides | and | Lactobacillus alimentarius, | which had relatively strong activity against | Pseudomonas aeruginosa | and | Bacillus subtilis | . |
[42] |
|
Leuconostocaecae |
Leuconostoc |
+ |
- |
- |
Changeable |
D |
| 30 dairy samples (household milk and curd) |
12 | Lactobacillus | isolates (LBS 1-LBS 12) |
* Eight isolates (LBS 1-6, 8 and 11) were bile resistant (survival >50% at 0.3% bile salt | w | / | v | ) and five isolates (LBS 1, 2, 5, 6 and 11) were resistant at acidic pH (survival >50% at pH 3).
|
Nondairy |
|
|
|
|
|
|
|
| |
Aerococcaceae |
Aerococcus |
+ |
- |
- |
- |
L |
| |
Carnobacteriaceae |
Carnobacterium |
+ |
- |
- |
NA |
L |
| |
Enterococcaceae |
Tetragenococcus |
+ |
- |
+ |
Changeable |
L |
| |
Enterococcaceae |
Vagococcus |
+ |
- |
- |
NA |
L |
| |
|
Fructobacillus |
+ |
NA |
- |
NA |
D |
| |
Leuconostocaecae |
Oenococcus |
+ |
- |
- |
Changeable |
D |
| |
|
Weissella |
+ |
- |
- |
Changeable |
D, L |
NA: Not available, D: Dextrorotary; optical rotation to the right (+), L: Levorotary; optical rotation to the left (−).
3. Importance of LABs in Dairy Foods in Terms of Health
LAB play a key role in the positive health effects of fermented milks and dairy products. LAB can sometimes be found naturally in some dairy products, or they can be added as a starter culture or sometimes novel ingredients/additives for increasing the functionality especially for probiotic potential of the product even if there are some unclear matters. LAB are a group of bacteria often found in fermented dairy foods that include genera such as
Lactobacillus,
Lactococcus,
Pediococcus,
Enterococcus, and
Streptococcus [21]. Most bacteria used as probiotics belong to the LAB species, genus
Lactobacillus (
Lactobacillus acidophilus,
Limosilactobacillus fermentum,
Lacticaseibacillus casei,
Limosilactobacillus reuteri,
Lactocaseibacillus rhamnosus,
Lactobacillus helveticus,
Lactococcus lactis,
Lactobacillus crispatus,
Lactobacillus gasseri, and
Lactiplantibacillus plantarum) and genus
Enterococcus (
Enterococcus faecalis and
Enterococcus faecium)
[22]. Since LAB starter cultures have been used for many years to carry out food and milk fermentation, these bacteria are generally considered ‘generally recognized as safe’(GRAS)
[23].
The main factors affecting the nutritional value of dairy products are the milk-based sources used (animal type, diet, age, lactation period, etc.) and food processing processes (temperature, storage conditions, etc.). In addition, starter cultures and/or probiotic types used in fermentation are a factor that directly affects the nutritional value of fermented milk products
[24]. Probiotics are generally divided into three groups: LAB-based traditional probiotics, non-LAB probiotics, and next-generation probiotics. Although other microorganisms are used in fermented dairy products, LAB has a greater place. The main dairy products in which LAB are used fermented milk, yogurt, infant formula, cheese, butter and cream, and ice cream
[25][26][25,26]. In general, dairy products are considered primary dietary sources for LABs as probiotics which can be found naturally or added afterwards
[27]. Although there is no certain cell count level that can guarantee the health effects of the probiotic strain in a food product, at least 10
6–10
8 cfu/g is reported as a sufficient amount to benefit from the beneficial effects of probiotics
[25]. This clearly shows that the presence of a culture that can show probiotic potentials does not guarantee that the product will be probiotic. Many factors affect the viability and stability of probiotics in dairy products. These are titratable acidity, pH, homogenisation, dissolved oxygen content, H
2O
2, storage temperature, type of probiotic, lactic acid concentration, and species and strains of the associative organism
[28]. However, some methods have been developed to preserve the viability of probiotics. In this context, there are studies to increase probiotic viability with microencapsulation and prebiotic addition techniques
[28][29][28,29].
In fermented milk products, bacteria belonging to
Lactobacillus,
Lactococcus,
Leuconostoc,
Pediococcus,
Bacillus,
Propionibacterium, and
Bifidobacterium genera are mostly prominent
[30].
Lactobacillus genus is the largest genus with 314 species and has become the most associated keyword with probiotics in the last 20 years. However, there is no consensus among scientists about the acceptance of some LAB genus as probiotics
[31]. Some of the most studied probiotic LAB in the literature are
Lactocaseibacillus rhamnosus GG (ATCC53103),
Lactocaseibacillus rhamnosus HN001,
Lacticaseibacillus casei Shirota,
Lacticaseibacillus casei Zhang,
Lactobacillus acidophilus NCFM,
Lactobacillus acidophilus LA-5, and
Lacticaseibacillus casei DN-114 001. Recently, novel probiotic LAB such as
Limosilactobacillus reuteri and
Lactobacillus johnsonii are used in the development of functional dairy products
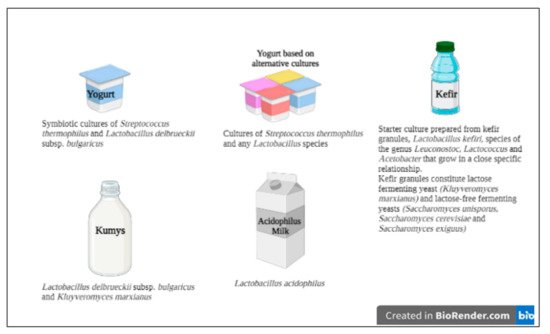
[25][32][25,32]. According to the Codex Alimentarius definitions of milk and dairy products, the specific starter cultures of yogurt and other fermented milk products are given in
Figure 1 [33]. Since the beneficial effects of probiotics are strain-specific, different strains of the same species can cause completely different effects on the host. Thus, it is stated that more studies are needed to understand the probiotic potential of new LAB strains and also well-known starter cultures of dairy products
[34].
| * All isolates inhibited the growth of |
| Staphylococcus aureus. |
|
| * LBS 2 also inhibited the growth of |
| Escherichia coli |
| and |
| Salmonella typhimurium |
| . |
| * Isolate LBS 2 was resistant to five antibiotics as well as | Lactocaseibacillus rhamnosus | LBS2 successfully adhered to rat epithelial cells in in vitro conditions. |
[43] |
| Traditional Greek dairy products (Feta, Kasseri, Xynotyri, Graviera, Formaela, Galotyri, and Kefalotyri cheeses as well as yogurt and milk) |
25 LAB strains |
* Only | Streptococcus thermophilus | ACA-DC 26 (Greek yogurt isolate) had antimicrobial activity (against | Streptococcus mutans | LMG 14558 | T | ).
* Two | Lactiplantibacillus plantarum | strains (ACA-DC 2640 and ACA-DC 4039) showed the highest adhesion according to a collagen-based microplate assay and by using HΤ-29 and Caco-2 cells.
* Milk cell-free supernatants of | Lactiplantibacillus |
| plantarum | ACA-DC 2640 and ACA-DC 4039 showed strong angiotensin I-converting enzyme inhibition.
* | Lactiplantibacillus plantarum | ACA-DC 2640, | Streptococcus thermophilus | ACA-DC 26, and ACA-DC 170 had anti-inflammatory activity. |
[44] |
| Tibetan kefir |
Lactobacillus kefiranofaciens | XL10 |
* XL10 survived 3-h incubation at pH 3.5 and exhibited cell surface hydrophobicity of ~79.9% and autoaggregation of ~27.8%.
* XL10 successfully adhered to the mucous tissue and colonized the ileum of the mice.
* XL10 modulated gut microbiota by increasing the | Bifidobacteriaceae | family and decreasing in Proteobacteria phyla. |
[45] |
| Mongolian fermented koumiss |
Lactobacillus helveticus | NS8 |
* Although NS8 exhibited a moderate survival ability in the gastrointestinal tract environment in vitro, an excellent adhesion ability to human intestinal cells and significant autoaggregation and cell-surface hydrophobicity were reported.
* NS8 was able to decline the proinflammatory effects of lipopolysaccharide by inducing higher levels of IL-10. |
[38] |
LAB: Lactic acid bacteria, EPS: Exopolysaccharides.4. Gut–Brain Axis of Dairy LABs.
As a result of significant research on human microbiota and probiotics in recent years, the gut has gained a new reputation as a “second brain” due to its microflora
[46]. Undoubtedly, the discovery of the gut–brain axis underlies this. The gut–brain axis (HPA) is a bidirectional communication network that connects the enteric and central nervous systems. This network is not only anatomical but also includes endocrine, humoral/metabolic, and immune communication pathways
[47][48][47,48]. This communication network includes the central nervous system (CNS), both the brain and spinal cord, the autonomic nervous system (ANS), the enteric nervous system (ENS), as well as the hypothalamic–pituitary–adrenal axis (HPA), thus connecting the gut and the brain to each other
[11][47][11,47]. It is suggested that the communication network established between the gut and the brain in this way may be related to many important health parameters such as intestinal activities and gastrointestinal function, metabolic diseases, cognitive performance, and mental health
[46][47][46,47].
The gut microbiota has the ability to modulate the ENS and CNS, with the ability to produce many neurotransmitters, as in the human brain
[49]. Among the neurotransmitters produced locally by the gut microbiota, there are local neurotransmitters such as γ-aminobutyric acid (GABA), noradrenaline, dopamine, serotonin (5-hydroxytryptamine-5-HT), melatonin, histamine and acetylcholine, and a biologically active catecholamine form in the lumen, thereby can affect the nervous system activity
[50][51][52][50,51,52]. This modulation is provided through many mechanisms including the vagus nerve and HPA and inflammatory cytokines as well as neurotransmitters
[53]. In addition, the production of some molecules, such as nitric oxide, which interacts with the vanilloid receptor on capsaicin-sensitive nerve fibres by some specific microbes such as
Lactobacilli, contributes to this modulation
[54]. This modulation is influenced by genetics, lifestyle habits (nutrition, drug use, exercise, etc.), and many environmental factors (stress, fear, social interaction, etc.), and the composition of the intestinal microbiota plays a key role in this modulation
[49]. In fact, as the evidence on the effectiveness of diet in the intestinal microbiota increases in recent years, the “gut–brain axis” network, known as bi-directional communication, will be started to be called three-directional as the “food–gut–brain axis”
[48][55][48,55].
The limited studies on humans related to this axis are mostly conducted on LAB and are often associated with mental performance, psychological and related immunological parameters
[47][56][57][47,56,57]. It was determined that a fermented milk product containing
Lactobacillus helveticus IDCC3801 improved cognitive performance after 12 weeks in healthy older adults (60–75 years)
[56]. In another study, the regular consumption of the milk product fermented with
Lacticaseibacillus casei DN-114001 and yogurt culture for 6 weeks modulated the immune response (lymphocyte and CD56 cell count) of university students under academic stress was reported
[57]. It is also found that the
Lactobacillus increased after consumption of unpasteurized milk and products (without comparison with pasteurized milk as control), and microbiome profile and function and the level of faecal valerate, as determined by measuring estimated neuroactive potential using a gut–brain modulation approach are increased
[12]. Thus, the results of all these researches showed that LAB originating from milk and dairy products play a key role in this axis and thus are related to health parameters. However, no correlation between LAB counts and mental and psychological measures was reported. Hence, it can be said that the evidence for a direct effect of dairy LAB on the gut–brain axis is still lacking.