A scaffold is a crucial biological substitute designed to aid the treatment of damaged tissue caused by trauma and disease. Various scaffolds are developed with different materials, known as biomaterials, and have shown to be a potential tool to facilitate in vitro cell growth, proliferation, and differentiation. Among the materials studied, carbon materials are potential biomaterials that can be used to develop scaffolds for cell growth. Many researchers have attempted to build a scaffold following the origin of the tissue cell by mimicking the pattern of their extracellular matrix (ECM). In addition, extensive studies were performed on the various parameters that could influence cell behaviour. Previous studies have shown that various factors should be considered in scaffold production, including the porosity, pore size, topography, mechanical properties, wettability, and electroconductivity, which are essential in facilitating cellular response on the scaffold. These interferential factors will help determine the appropriate architecture of the carbon-based scaffold, influencing stem cell (SC) response.
- carbon-based
- scaffold
- biomaterial
- biophysical factors
- stem cells
- tissue engineering
1. Carbon as Scaffold Biomaterials for SC Applications
| Types of Carbon | Dimensions | Composite Material |
Fabrication Methods | Types of Cells | Ref. |
|---|---|---|---|---|---|
| Carbon Nanocage | 3D nanoscale | - | - | HUC-MSCs | [10] |
| Fullerene | Aligned fullerene nanowhisker nanopatterned | - | Langmuir–Blodgett | Human MSCs | [11] |
| Aligned fullerene nanowhiskers | - | Modified liquid–liquid interfacial precipitation method | NSCs | [12] | |
| Graphene | rGONRs grids | polydimethylsiloxane (PDMS) | Drop casting method | Human MSCs | [13] |
| 2D graphene (GNOs, GONRs, GONPs) | distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino (polyethylene glycol)] (DSPE-PEG) | GONRs synthesis by using modified longitudinal unzipping method; GONPs synthesis by using modified Hummer’s method | AMSCs, BMSCs | [14] | |
| 3D matrix | Polycaprolactone (PCL) | Extrusion-based additive manufacturing | AMSCs | [3] | |
| 3D foams and 2D films | - | chemical vapor deposition (CVD) | NSCs | [15] | |
| Fibres | Poly-L-lactic-acid (PLLA) | Thermal-induced phase separation | BMSCs | [16] | |
| Nanosheets | PCL | Water-assisted liquid phase exfoliation | AMSCs | [17] | |
| 3D graphene oxide | Polypeptide thermogel | Temperature-sensitive sol to gel transition | Tonsil-derived MSCs | [18] | |
| 3D graphene | Nickel foam | CVD | Mouse NSCs | [19] | |
| 3D Graphene/SWCNT | - | CVD | Mouse MSCs | [20] | |
| Carbon Nanotube | COOH-SWCNT and -MWCNT, PEG-SWCNT | Ethanol, polyethylene Glycol (PEG) | Air brush spraying on a coverslip | Canine MSCs | [5] |
| CNT fibres | PLLA | Thermal-induced phase separation | BMSCs | [16] | |
| CNT | PCL | CVD | AMSCs | [17] | |
| MWCNT | PCL | Electrospinning | Human Dental Pulp Stem Cell | [21] | |
| MWCNT | Thermoplastic polyurethane | Electrospinning | Rat AMSCs | [22] | |
| MWCNT | PLLA | Electrospinning | Mouse ESCs | [23] | |
| MWCNT | Collagen hydrogel | Gelation | Rat MSCs | [24] | |
| MWCNT | Polyion complex hydrogel | Extrusion-based 3D printing | Rat BMSCs | [25] | |
| MWCNT | PEG | Drop-drying method | Human MSCs | [26] | |
| MWCNT | Poly-lactic acid (PLA), alginate, gelatine | Layer-by-layer assembly method | Wharton’s Jelly-derived mesenchymal stem cells (WJMSCs) | [27] | |
| Nanodiamond | Monolayer | - | Ultrasonication | Human NSCs | [28] |
| Reticulated vitreous carbon | 3D Foam | - | Etching and Pyrolysis | BMSCs | [29] |
| Carbon Nano-onions | Poly 4-mercaptophenyl methacrylate-carbon nano-onions | PCL | Probe sonication, hydraulic pressing | Human osteoblast cells | [30] |
| Oxidized CNOs | Chitosan, poly(vinyl-alcohol) | Cure on acetate molds | In vivo study on Wistar rat | [31] | |
| Poly 4-mercaptophenyl methacrylate-carbon nano-onions | Bovine serum albumin, trifluoroacetic acid | Force spinning | Human fibroblast cells | [32] | |
| Poly 4-mercaptophenyl methacrylate-carbon nano-onions | Gelatin | Probe sonication, freeze drying | Human osteoblast cells | [33] | |
| Carbon black nanoparticle | Nanoparticles | - | Probe sonication | In vivo study on mouse brains astrocyte | [34] |
| Carbon dots | Citric acid-derived nanodots | - | Hydrothermal | Rat BMSCs | [35] |
| Porphyra polysaccharide-derived carbon dots | - | Hydrothermal | Ectodermal MSCs | [36] | |
| Cellulose-derived reduced nanographene oxide carbon nanodots | PCL | Microwave | MG63 | [37] | |
| Onion-derived carbon nanodots | - | Microwave | Human foreskin fibroblast, MG63, red blood cells | [38] | |
| Human fingernail-derived carbon nanodots | - | Pyrolysis | HEK-293 | [39] | |
| Food-derived carbon nanodots | Glass beads | Hydrothermal | Prostate cancer (PC3) cells, NRK cells | [40] |
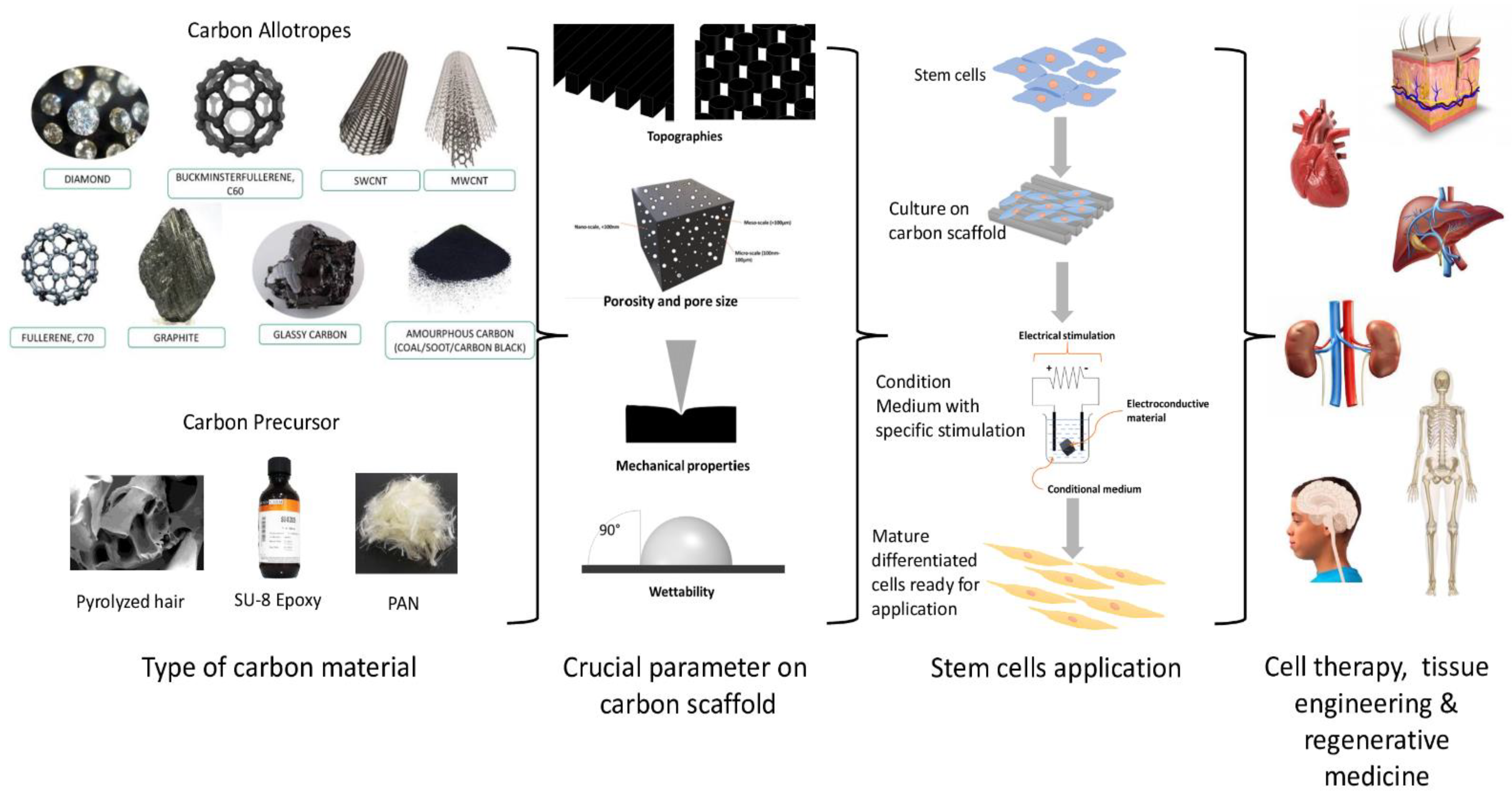
2. Carbon Precursors as Scaffold Biomaterials for SCs Applications
The use of carbon precursors as an alternative to carbon has shown more advantages and has received much attention in the production of carbon-based microstructures. In the last few years, the fabrication of carbon precursors, in various studies due to their manufacturing flexibility and customizable properties, has been employed [43]. Carbon precursor materials can be converted into high-percentage carbon materials when subjected to high temperatures or chemicals. The tuneability properties of carbon precursors have allowed the production of different patterns (i.e., organised or random alignment) that mimic the microenvironment niche of cells by using a simple method with good reproducibility and low cost. Plus, carbon precursors can be tailored to produce conductive carbon-based scaffolds through modifications to their chemical composition and pyrolysis process (Table 2). Every scaffold should exhibit mechanically and biologically suitable qualities, mimicking the ECM of SCs to support the adhesion and development of cells, something which carbon precursors can provide [44][52].
Table 2.
Carbon precursor in biological applications.
|
Type of Precursor |
Fabrication Method |
Structure |
Application |
Ref. |
|
Citric acid |
Hydrothermal |
Carbon nanodots |
Rat BMSCs |
[45] |
|
Porphyra polysaccharide |
Hydrothermal |
Carbon nanodots |
Ectodermal MSCs |
[46] |
|
Polyacrylonitrile (PAN) |
Electrospun, pyrolysis |
Electrospun carbon nanofibres |
Mouse NSCs culture |
[47] |
|
Electrospun, pyrolysis |
Electrospun carbon nanofibres |
Human endometrial stem cells (hEnSCs) |
[48] |
|
|
Cryogel (chitosan/agarose/gelatin) |
Pyrolysis |
3D carbon-based scaffold |
NSCs |
[49] |
|
Sucrose |
Sugar blowing technique, Pyrolysis |
3D glassy carbon |
SH-SY5Y, HEK-293 |
[50] |
|
Polydopamine |
Electrospun, pyrolysis |
Microfibre scaffold |
NSCs |
[51] |
|
SU-8 |
Photolithography, pyrolysis |
3D carbon-based scaffold |
Human NSCs, PC12 |
[52] |
|
photolithography, Pyrolysis |
Gold nanoparticles glassy carbon |
Primary dermal fibroblast |
[53] |
|
|
Zif-8 |
Pyrolysis |
C-ZnO nanoparticles |
MSCs |
[54] |
|
Cotton |
Pyrolysis |
Pyrolysed cotton microfibres |
PC12 |
[55] |
|
Epoxy resin |
Stereolithography, pyrolysis |
Carbon microlattices |
MC3E3-E1 |
[56] |
3. Application of Carbon-Based Scaffold in Tissue Engineering
3.1. Neural Tissue
3.2. Cardiac Tissue
3.3. Bone Tissue
3.4. Others Tissue
4. Conclusions and Future Perspective
References
- Mishra, R.; Pramanick, B.; Maiti, T.K.; Bhattacharyya, T.K. Glassy carbon microneedles—new transdermal drug delivery device derived from a scalable C-MEMS process. Microsyst. Nanoeng. 2018, 4, 1–11.
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295.
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992.
- Fan, L.; Ma, R.; Zhang, Q.; Jia, X.; Lu, B. Graphite Anode for a Potassium-Ion Battery with Unprecedented Performance. Angew. Chem. Int. Ed. 2019, 58, 10500–10505.
- Das, K.; Madhusoodan, A.; Mili, B.; Kumar, A.; Saxena, A.C.; Kumar, K.; Sarkar, M.; Singh, P.; Shrivastava, S.; Bag, S. Functionalized carbon nanotubes as suitable scaffold materials for proliferation and differentiation of canine mesenchymal stem cells. Int. J. Nanomed. 2017, ume 12, 3235–3252.
- Shuai, C.; Li, Y.; Wang, G.; Yang, W.; Peng, S.; Feng, P. Surface modification of nanodiamond: Toward the dispersion of reinforced phase in poly-l-lactic acid scaffolds. Int. J. Biol. Macromol. 2019, 126, 1116–1124.
- Umeyama, T.; Imahori, H. Isomer Effects of Fullerene Derivatives on Organic Photovoltaics and Perovskite Solar Cells. Acc. Chem. Res. 2019, 52, 2046–2055.
- Siddique, A.B.; Pramanick, A.K.; Chatterjee, S.; Ray, M. Amorphous Carbon Dots and their Remarkable Ability to Detect 2,4,6-Trinitrophenol. Sci. Rep. 2018, 8, 9770.
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 2017, 295, 32–40.
- Zhai, L.; Maimaitiming, Z.; Cao, X.; Xu, Y.; Jin, J. Nitrogen-doped carbon nanocages and human umbilical cord mesenchymal stem cells cooperatively inhibit neuroinflammation and protect against ischemic stroke. Neurosci. Lett. 2019, 708, 134346.
- Song, J.; Jia, X.; Minami, K.; Hill, J.; Nakanishi, J.; Shrestha, L.K.; Ariga, K. Large-Area Aligned Fullerene Nanocrystal Scaffolds as Culture Substrates for Enhancing Mesenchymal Stem Cell Self-Renewal and Multipotency. ACS Appl. Nano Mater. 2020, 3, 6497–6506.
- Hsieh, F.-Y.; Shrestha, L.K.; Ariga, K.; Hsu, S.-H. Neural differentiation on aligned fullerene C60 nanowhiskers. Chem. Commun. 2017, 53, 11024–11027.
- Akhavan, O.; Ghaderi, E.; Shahsavar, M. Graphene nanogrids for selective and fast osteogenic differentiation of human mesenchymal stem cells. Carbon 2013, 59, 200–211.
- Talukdar, Y.; Rashkow, J.T.; Lalwani, G.; Kanakia, S.; Sitharaman, B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials 2014, 35, 4863–4877.
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604.
- Duan, S.; Yang, X.; Mei, F.; Tang, Y.; Li, X.; Shi, Y.; Mao, J.; Zhang, H.; Cai, Q. Enhanced osteogenic differentiation of mesenchymal stem cells on poly(l-lactide) nanofibrous scaffolds containing carbon nanomaterials. J. Biomed. Mater. Res. Part A 2015, 103, 1424–1435.
- Wang, W.; Huang, B.; Byun, J.J.; Bártolo, P. Assessment of PCL/carbon material scaffolds for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 93, 52–60.
- Patel, M.; Moon, H.J.; Ko, D.Y.; Jeong, B. Composite System of Graphene Oxide and Polypeptide Thermogel As an Injectable 3D Scaffold for Adipogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 5160–5169.
- Ma, Q.; Yang, L.; Jiang, Z.; Song, Q.; Xiao, M.; Zhang, D.; Ma, X.; Wen, T.; Cheng, G. Three-Dimensional Stiff Graphene Scaffold on Neural Stem Cells Behavior. ACS Appl. Mater. Interfaces 2016, 8, 34227–34233.
- Yan, X.; Yang, W.; Shao, Z.; Yang, S.; Liu, X. Graphene/single-walled carbon nanotube hybrids promoting osteogenic differentiation of mesenchymal stem cells by activating p38 signaling pathway. Int. J. Nanomed. 2016, ume 11, 5473–5484.
- Cedillo, M.L.F.; Estrada, K.N.A.; Pozos-Guillen, A.; Murguia, J.; Vidal, M.A.; Cervantes-Uc, J.M.; Rosalesibanez, R.; Cauich-Rodríguez, J.V. Multiwall carbon nanotubes/polycaprolactone scaffolds seeded with human dental pulp stem cells for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2015, 27, 1–12.
- Pouladzadeh, F.; Katbab, A.A.; Haghighipour, N.; Kashi, E. Carbon nanotube loaded electrospun scaffolds based on thermoplastic urethane (TPU) with enhanced proliferation and neural differentiation of rat mesenchymal stem cells: The role of state of electrical conductivity. Eur. Polym. J. 2018, 105, 286–296.
- Kabiri, M.; Soleimani, M.; Shabani, I.; Futrega, K.; Ghaemi, N.; Ahvaz, H.H.; Elahi, E.; Doran, M.R. Neural differentiation of mouse embryonic stem cells on conductive nanofiber scaffolds. Biotechnol. Lett. 2012, 34, 1357–1365.
- Li, S.; Xu, Q.; Wang, Z.; Hou, W.; Hao, Y.; Yang, R.; Murr, L. Influence of cell shape on mechanical properties of Ti–6Al–4V meshes fabricated by electron beam melting method. Acta Biomater. 2014, 10, 4537–4547.
- Cui, H.; Yu, Y.; Li, X.; Sun, Z.; Ruan, J.; Wu, Z.; Qian, J.; Yin, J. Direct 3D printing of a tough hydrogel incorporated with carbon nanotubes for bone regeneration. J. Mater. Chem. B 2019, 7, 7207–7217.
- Zhao, C.; Andersen, H.; Ozyilmaz, B.; Ramaprabhu, S.; Pastorin, G.; Ho, H.K. Spontaneous and specific myogenic differentiation of human mesenchymal stem cells on polyethylene glycol-linked multi-walled carbon nanotube films for skeletal muscle engineering. Nanoscale 2015, 7, 18239–18249.
- Ghorbani, S.; Tiraihi, T.; Soleimani, M. Differentiation of mesenchymal stem cells into neuron-like cells using composite 3D scaffold combined with valproic acid induction. J. Biomater. Appl. 2017, 32, 702–715.
- Taylor, A.C.; González, C.H.; Miller, B.; Edgington, R.J.; Ferretti, P.; Jackman, R.B. Surface functionalisation of nanodiamonds for human neural stem cell adhesion and proliferation. Sci. Rep. 2017, 7, 1–11.
- Wei, X.; Zhao, D.; Wang, B.; Wang, W.; Kang, K.; Xie, H.; Liu, B.; Zhang, X.; Zhang, J.; Yang, Z. Tantalum coating of porous carbon scaffold supplemented with autologous bone marrow stromal stem cells for bone regeneration in vitro and in vivo. Exp. Biol. Med. 2016, 241, 592–602.
- Mamidi, N.; Gamero, M.R.M.; Villela-Castrejón, J.; Elías-Zúñiga, A. Development of ultra-high molecular weight polyethylene-functionalized carbon nano-onions composites for biomedical applications. Diam. Relat. Mater. 2019, 97, 107435.
- Tovar, C.D.G.; Castro, J.I.; Valencia, C.H.; Porras, D.P.N.; Hernandez, J.H.M.; Valencia, M.E.; Velasquez, J.D.; Chaur, M.N. Preparation of Chitosan/Poly(Vinyl Alcohol) Nanocomposite Films Incorporated with Oxidized Carbon Nano-Onions (Multi-Layer Fullerenes) for Tissue-Engineering Applications. Biomolecules 2019, 9, 684.
- Mamidi, N.; Delgadillo, R.M.V.; González-Ortiz, A. Engineering of carbon nano-onion bioconjugates for biomedical applications. Mater. Sci. Eng. C 2021, 120, 111698.
- Mamidi, N.; Castrejón, J.V.; González-Ortiz, A. Rational design and engineering of carbon nano-onions reinforced natural protein nanocomposite hydrogels for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103696.
- Onoda, A.; Takeda, K.; Umezawa, M. Dose-dependent induction of astrocyte activation and reactive astrogliosis in mouse brain following maternal exposure to carbon black nanoparticle. Part. Fibre Toxicol. 2017, 14, 1–16.
- Shao, D.; Lu, M.; Xu, D.; Zheng, X.; Pan, Y.; Song, Y.; Xu, J.; Li, M.; Zhang, M.; Li, J.; et al. Carbon dots for tracking and promoting the osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2017, 5, 1820–1827.
- Chen, J.; Wang, Q.; Zhou, J.; Deng, W.; Yu, Q.; Cao, X.; Wang, J.; Shao, F.; Li, Y.; Ma, P. Porphyra polysaccha-ride-derived carbon dots for non-viral co-delivery of different gene combinations and neuronal differentiation of ectodermal mesenchymal stem cells. Nanoscale 2017, 9, 10820–10831.
- Erdal, N.B.; Hakkarainen, M. Construction of Bioactive and Reinforced Bioresorbable Nanocomposites by Reduced Nano-Graphene Oxide Carbon Dots. Biomacromolecules 2018, 19, 1074–1081.
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Das, B.; Mitra, A.; Dhara, S. Onion derived carbon nanodots for live cell imaging and accelerated skin wound healing. J. Mater. Chem. B 2017, 5, 6579–6592.
- Chatzimitakos, T.; Kasouni, A.I.; Troganis, A.N.; Stalikas, C.D. Carbonization of Human Fingernails: Toward the Sustainable Production of Multifunctional Nitrogen and Sulfur Codoped Carbon Nanodots with Highly Luminescent Probing and Cell Proliferative/Migration Properties. ACS Appl. Mater. Interfaces 2018, 10, 16024–16032.
- Xie, Y.; Nurkesh, A.; Keneskhanova, Z.; Altaikyzy, A.; Fan, H. Application of Electrically Charged Carbon in DNA and Cell Engineering. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; Volume 2018, pp. 4202–4204.
- Van der Sanden, B.; Dhobb, M.; Berger, F.; Wion, D. Optimizing stem cell culture. J. Cell. Biochem. 2010, 111, 801–807.
- Rokh, M.T.; Kouzani, A.Z.; Francis, P.; Kanwar, J.R. Microfluidic devices for cell cultivation and proliferation. Biomicrofluidics 2013, 7, 051502.
- Mishra, R.; Pramanick, B.; Maiti, T.K.; Bhattacharyya, T.K. Glassy carbon microneedles—new transdermal drug delivery device derived from a scalable C-MEMS process. Microsystems Nanoeng. 2018, 4, 1–11, doi:10.1038/s41378-018-0039-9.
- Eyni, H.; Ghorbani, S.; Shirazi, R.; Salari Asl, L.; P Beiranvand, S.; Soleimani, M. Three-dimensional wet-electrospun poly (lactic acid)/multi-wall carbon nanotubes scaffold induces differentiation of human menstrual blood-derived stem cells into germ-like cells. J. Biomater. Appl. 2017, 32, 373–383, doi:10.1177%2F0885328217723179.
- Shao, D.; Lu, M.; Xu, D.; Zheng, X.; Pan, Y.; Song, Y.; Xu, J.; Li, M.; Zhang, M.; Li, J.; et al. Carbon dots for tracking and promoting the osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2017, 5, 1820–1827, doi:10.1039/c7bm00358g.
- Chen, J.; Wang, Q.; Zhou, J.; Deng, W.; Yu, Q.; Cao, X.; Wang, J.; Shao, F.; Li, Y.; Ma, P. Porphyra polysaccha-ride-derived carbon dots for non-viral co-delivery of different gene combinations and neuronal differentiation of ectodermal mesenchymal stem cells. Nanoscale 2017, 9, 10820–10831.
- Zhu, W.; Ye, T.; Lee, S.-J.; Cui, H.; Miao, S.; Zhou, X.; Shuai, D.; Zhang, L.G. Enhanced neural stem cell functions in con-ductive annealed carbon nanofibrous scaffolds with electrical stimulation. Nanomedicine: Nanotechnology, Biol. Med. 2018, 14, 2485–2494, doi:10.1016/j.nano.2017.03.018.
- Mirzaei, E.; Ai, J.; Ebrahimi-Barough, S.; Verdi, J.; Ghanbari, H.; Faridi-Majidi, R. The Differentiation of Human Endometrial Stem Cells into Neuron-Like Cells on Electrospun PAN-Derived Carbon Nanofibers with Random and Aligned Topographies. Mol. Neurobiol. 2016, 53, 4798–4808, doi:10.1007/s12035-015-9410-0.
- Fuhrer, E.; Bäcker, A.; Kraft, S.; Gruhl, F.J.; Kirsch, M.; MacKinnon, N.; Korvink, J.; Sharma, S. 3D Carbon Scaffolds for Neural Stem Cell Culture and Magnetic Resonance Imaging. Adv. Health Mater. 2017, 7, doi:10.1002/adhm.201700915.
- Tadyszak, K.; Litowczenko, J.; Majchrzycki, Łukasz; Jeżowski, P.; Załęski, K.; Scheibe, B. Sucrose based cellular glassy carbon for biological applications. Mater. Chem. Phys. 2020, 239, 122033, doi:10.1016/j.matchemphys.2019.122033.
- Yang, Y.; Zhang, Y.; Chai, R.; Gu, Z. A Polydopamine-Functionalized Carbon Microfibrous Scaffold Accelerates the Development of Neural Stem Cells. Front. Bioeng. Biotechnol. 2020, 8, 616, doi:10.3389/fbioe.2020.00616.
- Amato, L.; Heiskanen, A.; Caviglia, C.; Shah, F.; Zór, K.; Skolimowski, M.; Madou, M.J.; Gammelgaard, L.; Hansen, R.; Seiz, E.G.; et al. Pyrolysed 3D-Carbon Scaffolds Induce Spontaneous Differentiation of Human Neural Stem Cells and Facilitate Real-Time Dopamine Detection. Adv. Funct. Mater. 2014, 24, 7042–7052, doi:10.1002/adfm.201400812.
- Chauhan, G.; Ángeles, A.L.; Gonzalez, E.G.; Kulkarni, M.M.; Cardenas‐Benitez, B.; Jiménez, M.F.; Santiago, G.T.; Alvarez, M.M.; Madou, M.; Martinez‐Chapa, S.O. Nano‐spaced Gold on Glassy Carbon Substrate for Controlling Cell Behavior. Adv. Mater. Interfaces 2020, 7, 2000238, doi:10.1002/admi.202000238.
- Xia, Y.; Fan, X.; Yang, H.; Li, L.; He, C.; Cheng, C.; Haag, R. ZnO/Nanocarbons‐Modified Fibrous Scaffolds for Stem Cell‐Based Osteogenic Differentiation. Small 2020, 16, 2003010, doi:10.1002/smll.202003010.
- Chen, X.; Wu, Y.; Ranjan, V.D.; Zhang, Y. Three-dimensional electrical conductive scaffold from biomaterial-based carbon microfiber sponge with bioinspired coating for cell proliferation and differentiation. Carbon 2018, 134, 174–182, doi:10.1016/j.carbon.2018.03.064.
- Islam, M.; Lantada, A.D.; Gómez, M.R.; Mager, D.; Korvink, J. Microarchitectured Carbon Structures as Innovative Tissue Engineering Scaffolds. Adv. Eng. Mater. 2020, 22, 2000083, doi:10.1002/adem.202000083.
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.-N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.-W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072.
- Hasanzadeh, E.; Ebrahimibarough, S.; Mirzaei, E.; Azami, M.; Tavangar, S.M.; Mahmoodi, N.; Basiri, A.; Ai, J. Preparation of fibrin gel scaffolds containing MWCNT/PU nanofibers for neural tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 802–814.
- Lee, S.-J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural Eng. 2018, 15, 016018.
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287.
- Yan, C.; Ren, Y.; Sun, X.; Jin, L.; Liu, X.; Chen, H.; Wang, K.; Yu, M.; Zhao, Y. Photoluminescent functionalized carbon quantum dots loaded electroactive Silk fibroin/PLA nanofibrous bioactive scaffolds for cardiac tissue engineering. J. Photochem. Photobiol. B Biol. 2020, 202, 111680.
- Martinelli, V.; Bosi, S.; Peña, B.; Baj, G.; Long, C.S.; Sbaizero, O.; Giacca, M.; Prato, M.; Mestroni, L.; Peña, B. 3D Carbon-Nanotube-Based Composites for Cardiac Tissue Engineering. ACS Appl. Bio Mater. 2018, 1, 1530–1537.
- Tohidlou, H.; Shafiei, S.S.; Abbasi, S.; Asadi-Eydivand, M.; Fathi-Roudsari, M. Amine-functionalized Single-walled Carbon Nanotube/Polycaprolactone Electrospun Scaffold for Bone Tissue Engineering: In vitro Study. Fibers Polym. 2019, 20, 1869–1882.
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337.
- Shafiei, S.; Omidi, M.; Nasehi, F.; Golzar, H.; Mohammadrezaei, D.; Rad, M.R.; Khojasteh, A. Egg shell-derived calcium phosphate/carbon dot nanofibrous scaffolds for bone tissue engineering: Fabrication and characterization. Mater. Sci. Eng. C 2019, 100, 564–575.
- Amiryaghoubi, N.; Pesyan, N.N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357.
- Dai, C.; Li, Y.; Pan, W.; Wang, G.; Huang, R.; Bu, Y.; Liao, X.; Guo, K.; Gao, F. Three-Dimensional High-Porosity Chitosan/Honeycomb Porous Carbon/Hydroxyapatite Scaffold with Enhanced Osteoinductivity for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 575–586.
- Tondnevis, F.; Ketabi, M.A.; Fekrazad, R.; Sadeghi, A.; Keshvari, H.; Abolhasani, M.M. In Vitro Characterization of Polyurethane-Carbon Nanotube Drug Eluting Composite Scaffold for Dental Tissue Engineering Application. J. Biomim. Biomater. Biomed. Eng. 2020, 47, 13–24.
- Gopinathan, J.; Pillai, M.M.; Shanthakumari, S.; Gnanapoongothai, S.; Rai, B.K.D.; Sahanand, K.S.; Selvakumar, R.; Bhattacharyya, A. Carbon nanofiber amalgamated 3D poly-ε-caprolactone scaffold functionalized porous-nanoarchitectures for human meniscal tissue engineering: In vitro and in vivo biocompatibility studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2247–2258.
- Stocco, T.D.; Antonioli, E.; Romagnolli, M.L.; Sousa, G.; Ferretti, M.; Lobo, A.O. Aligned biomimetic scaffolds based on carbon nanotubes-reinforced polymeric nanofibers for knee meniscus tissue engineering. Mater. Lett. 2020, 264, 127351.
- Yang, R.; Yang, S.; Li, K.; Luo, Z.; Xian, B.; Tang, J.; Ye, M.; Lu, S.; Zhang, H.; Ge, J. Carbon Nanotube Polymer Scaffolds as a Conductive Alternative for the Construction of Retinal Sheet Tissue. ACS Chem. Neurosci. 2021, 12, 3167–3175.
- Donnelly, H.; Salmeron-Sanchez, M.; Dalby, M.J. Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 2018, 15, 20180388.
- Pennings, S.; Liu, K.J.; Qian, H. The Stem Cell Niche: Interactions between Stem Cells and Their Environment. Stem Cells Int. 2018, 2018, 1–3.
