Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 3 by Nora Tang.
Some of the vital limitations to the broader use of these biopolymers are that they are less flexible and have less impact resistance when compared to petroleum-based plastics (e.g., polypropylene (PP), high-density polyethylene (HDPE) and polystyrene (PS)). Recent advances have shown that with appropriate modification methods—plasticizers and fillers, polymer blends and nanocomposites, such limitations of both polymers can be overcome. This work is meant to widen the applicability of both polymers by reviewing the available materials on these methods and their impacts with a focus on the mechanical properties.
- poly(lactic acid) (PLA)
- polyhydroxyalkanoates (PHAs)
- properties
1. PLA Foams, 3D-printed Scaffolds and Flame Retardancy
One of the ideal materials for various packaging applications is PLA foam. PLA foams are becoming increasingly desired as renewable biopolymer alternatives to petroleum-based polymer foams. This is due to their light weight and good cushioning properties. Polymers foams are produced using different blowing agents, which can be categorized as chemical and physical. The production of foam cells under the impact of pressure/temperature release is done via chemical blowing agents. On the other hand, physical foaming of polymers and composites, results in the production of cellular structures with cell sizes smaller than 10 µm and cell densities greater than 109 cells/cm3, known as microcellular foams [1][2]. PLA foams can be produced via batch processing, foam injection molding, bead foaming as well as foam extrusion. Foam PLA/natural (lignocellulosic) fiber-reinforced composites are produced mainly using foam injection molding and extrusion. Figure 1, Figure 2 and Figure 3, show the concept of foam injection molding, foam extrusion and bead foaming, respectively. Such foams exhibit thermal and mechanical properties comparable to those of the currently used petroleum-based foams [3][4]. An improvement of the morphology of the foam cell via modification of polymer melt viscosity is believed to be feasible through the addition of lignocellulosic fibers. For example, the incorporation of lignocellulosic fibers can lower the cell size and increase foam cell density [2][5][6][7][8][9][10]. Moreover, PLA/natural (lignocellulosic) fiber-reinforced composites were found to enhance specific tensile and flexural moduli. Furthermore, changes in PLA crystallinity prompted by the incorporation of fibers can potentially alter the foam cell characteristics of PLA composites [11].
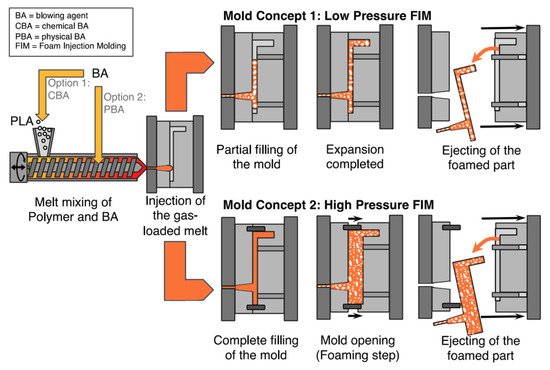
Figure 1. The concept of foam injection molding [3].
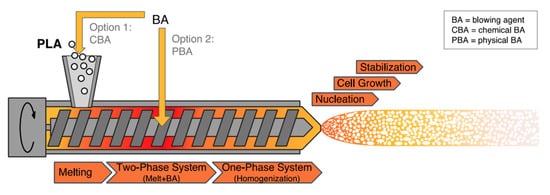
Figure 2. The concept of foam extrusion [3].
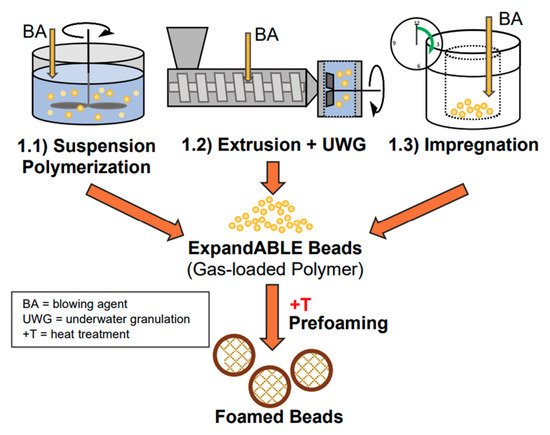
Figure 3. The various methods for the production of expandable bead foams [3].
In one study [11], microcellular injection molding process was used to produce foamed flax fiber reinforced PLA composites at three various flax concentrations (1, 10 and 20 wt.%). Neat PLA was reported to have an average cell size of 8.4 µm. On the other hand, the cell sizes of PLA/flax composites dropped by around 11%, 47% and 67% at 1, 10 and 20 wt.% flax fiber, respectively. The study has also reported an increase in the specific tensile modulus by around 3%, 10% and 22% for the foamed composites at 1, 10 and 20 wt.% fiber loadings, respectively. This was attributed to the flax fibers’ higher modulus in comparison to that of PLA as well as to the restraining impact of fillers on the movement of polymer chain yielding to enhanced stiffness [11].
An investigation of the impact of the addition of willow fiber at 20 wt.% and 30 wt.% concentrations on the mechanical properties of foamed PLA composites was done by Zafar et al. [12]. Neat PLA was reported to have an average cell size of about 33.7 µm. However, the cell sizes of PLA/flax composites dropped to about 20.6 µm at 20 wt.% and 18.1 µm at 30 wt.% willow fiber, respectively. The study reported a slight increase in the flexural modulus and the maximum value was corresponding to that of PLA/30 wt.% willow fiber composite. The specific notched impact strength of PLA/flax composites increased by about 16% at 20% and 45% at 30 wt.% willow fiber, respectively. Moreover, the degree of crystallinity has also increased with the addition of willow fiber [12].
In addition to their use in packaging applications, foamed PLA products have been also utilized in tissue engineering and drug release. PLA is often used in bone or cartilage tissue engineering in the form of 3D-printed scaffolds. One of the most widely used technologies for PLA is additive manufacturing. Generally, a precise control of the 3D printer is essential for the quality of 3D-printed products. A wide range of 3D printers such as Ultimakers and Robo can be used to 3D print PLA filaments [13]. The strength of PLA’s printed parts depends mainly on the direction of printing. Therefore, the following points should be given special considerations during the 3D printing. Force application’s direction shall not be perpendicular to the printing layer and when printing complex parts, the outer shell thickness, printing pattern, density and the interconnecting parts must be given great considerations as they can lead to premature brittleness. Another important point is to make sure that the platform holds the 3D-printed part firmly so as to avoid the printed spot from being distorted or pulled out. Therefore, it is recommended to use a painter’s tape to hold the platform firmly in position [14]. Using a painter’s tape will also make it easy to take off the PLA’s 3D-printed part as the printed object will stick to the surface of the painter’s tape. This will avoid damaging the object when the 3D printing is finished. Another benefit of using a painter’s tape is that it can help in avoiding warpage, particularly for semicrystalline PLA which can undertake substantial irregularity in shrinkage when molten PLA’s layers are laid continuously. Moreover, heating the platform can also create a sticking effect. Nevertheless, the platform’s temperature shall be kept within the limits that will not cause polymer softening or degradation. The recommended platform and printing temperatures for PLA are 60 °C and 210 °C, respectively. Exposing the PLA filament to high temperatures and moisture can lead to degradation, depolymerization and/or chain scissioning. Therefore, it is recommended to keep the PLA filament stored in a securely sealed condition at a relative humidity less than 10%. Unsealing of the PLA filament is recommended just before the start of printing.
Different studies in literature have reported producing medical implants at a more affordable cost using 3D printing of PLA. Conventional manufacturing techniques such as casting or forging are time consuming and most of the time fail to meet the patients’ needs. Three-dimensional printing of scaffolds is one of the mostly suggested medical applications for PLA. For these 3D-printed scaffolds to be able to offer an interconnected network for cell growth as well as transportation of nutrients and waste generated from metabolism, they must meet certain mechanical properties, structural features and durability. Furthermore, these scaffolds are biocompatible with controlled rate of degradation Therefore, in the long term, there should be no problem for these scaffolds to adhere and match with the tissues [15]. According to Kikuchi et al. [16], to meet the functional requirements of scaffolds, bioactive ceramics such as beta-tricalcium phosphate, hydroxyapatite and calcium phosphate are incorporated with PLA [16]. Niaza et al. [17] investigated 3D printing of porous scaffolds of compounded hydroxyapatite and PLA at an average particle size of 90 nm and 1 μm. FFF technique with a nozzle temperature of 220 °C was used. Results suggested that the modulus of elasticity for PLA with micro-sized hydroxyapatite and nano-sized hydroxyapatite were 2.8 and 4.0 GPa respectively. Knowing that the Young’s modulus for the trabecular bone is in the range of 3–5 GPa, it is then very likely to use the 3D-printed PLA- nanosized hydroxypatite composite bone scaffolding as an alternative to original bone as implants. Moreover, the formation of a high-porosity structure due to the sintering between the layers during 3D printing makes PLA-hydroxyapatite composite a good feasible substitute to original bone [17]. Generally, high porosity is linked to a weaker structure, which in this case weaker PLA-hydroxyapatite composite, nonetheless, such a condition is safe with the addition of nano-sized hydroxyapatite. Various porosities of 3D-printed PLA scaffold structures were studies and compared by Gregor et al. [18]. The investigators were able to 3D print scaffolds with various geometrical structures using FDM. Two types of scaffolds of the defined shape and engineered inner structure that provides regular and sufficient porosity have been successfully printed. The designed 3D-printed scaffolds were subjected to osteosarcoma cells proliferation experiment and mechanical testing. Results suggest that the proliferation of both types of 3D-printed scaffolds with porosity values of 30% and 50% was satisfying with good mechanical durability [18]. Figure 4 shows a schematic of production of filament as well as 3D-printed scaffolds using FFF. According to Alam et al. [19], the process involves (i) solvent casting followed by (ii) filaments fabrication via extrusion and finally (iii) 3D printing of scaffolds.

Figure 4. (a) Schematic of filament fabrication and 3D printing of scaffolds and (b) optical images of various scaffolds ((i) for neat PLA, (ii) PLA/PCL (50/50 wt.%), (iii) PLA/PCL/HNTs (50/50/1 wt.%), (iv) PLA/PCL/HNTs (50/50/3 wt.%), (v) PLA/PCL/HNTs (50/50/5 wt.%) and (vi) PLA/PCL/HNTs (50/50/7 wt.%)) [19].
Various applications (e.g., construction, automobile and electronics) requires high criteria for dripping combustions and flammability, which cannot be satisfied by neat PLA. Therefore, there have been some attempts to enhance PLA’s flame retardancy for compact and foamed forms of PLA. In one study [20], an enhancement in compact PLA’s flame retardancy was achieved by a synergistic mixture of ammonium polyphosphate (APP) with expandable graphite (EG), an eco-friendly flame retardant. With 15% of this intumescent flame retardant (APP/EG = 3:1), there was an increase in the Limiting Oxygen Index (LOI) from 22 to 36.5. The UL-94-V-0 classification was also reached [20]. In another study [21], the same burning behavior was reported using 30% of a mixture (3:2) of a novel hyperbranched polyamine charring agent (HPCA) together with APP. Tang et al. [22] reported good flame retardancy, anti-dripping effects and high LOI values with synergistic combinations of expanded graphite and aluminum hypophosphite [22]. Other studies were done to enhance foamed PLA’s flame retardancy with a phosphorous containing flame retardant, as well as graphene [23] or starch [24] as a charring agent. UL-94-V-0 classification was reported. Moreover, LOI was significantly increased and anti-dripping effects were observed.
Vadas and coworkers were able to incorporate a bio-based flame retardant into a PLA extrusion foam [25]. A combination of APP as an intumescent flame retardant and flame retardant treated cellulose (surface treatment with boric acid and diammonium phosphate) as a bio-based charring agent was used to lower PLA foams’ flammability. A multifunctional epoxy-based chain extender was utilized and, even at elevated additive loadings, a substantial expansion with void fractions higher than 90% was attainable with carbon dioxide as a blowing agent. With an additive content lower than 20%, superb flame retardancy (LOI of 31.5% and UL-94 V-0) was reported. Moreover, compared to the compact materials, the flame retardant synergism was less noticeable in the expanded foams. This is attributed to the enlarged contact surface as well as the flame retardant’s lower volume concentration [23][24].
In another investigation [26], a novel flame retardant and toughened bio-based (PLA)/glycidyl methacrylate-grafted natural rubber (GNR) composite was reported. The interfacial compatibility between PLA/GNR matrix and the charring ability of the PLA/GNR/SiAHP composites as well as the modified aluminum hypophosphite by silane (SiAHP) was enhanced to a certain extent due to the surface modification of AHP. The flame retardancy and toughness of the PLA/GNR/SiAHP composites were slightly greater than those of PLA/GNR/AHP composites. UL-94 V-0 rating and LOI of 26.50% were reported. The promising flame retardancy of the PLA/GNR/SiAHP composites was suggested to be due to the synergistic effect including condensed and gaseous phase flame-retardant mechanisms. High-performance flame-retardant PLA/GNR/SiAHP composites have great potential applications as alternatives to petroleum-based polymers in the building and automotive interior sectors [26].
Li et al. [27] developed a cooperative flame-retardant system based on natural intumescent-grafted bamboo charcoal (BC) and chitosan (CS) for PLA. Figure 5 shows a schematic diagram of the composite preparation and testing. The composite demonstrated minimal decline in strength properties and enhanced flame retardancy. CS as an adhesion promoter enhanced the interfacial compatibility between PLA and graft modified bamboo charcoal resulting in improving the tensile properties by 8.42% and 11.11%, respectively for the Young’s modulus and tensile strength. The study found that CS endorses the reorganization of the internal crystal structure. At 3 wt.% CS and 30 wt.% graft-modified bamboo charcoal, the composite’s crystallinity was reported to be 43 times that of neat PLA. Flammability tests (UL-94 V-0 rating and LOI of 33.6 vol.%) showed a substantial enhancement in flame retardancy. The reported composite is claimed to meet the requirements for strong, biodegradable and non-toxic PLA packaging products [27].
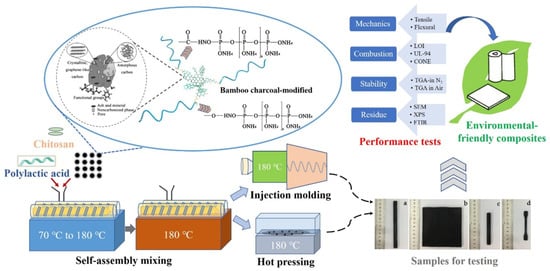
Figure 5. Composite preparation and testing as reported by Li et al. [27].
4.2. PHAs in Active Food Packaging
Technologies that are developed to enhance shelf life, sensory properties and keeps the packaged food safe from mechanical damage as well as microbial contamination are referred to as active food packaging. Bioactive agents (Figure 6) have been used to create edible films with induced desirable functionalities. Figure 7 shows a schematic of active food packaging based on bio nanocomposites with outstanding preservation capability against pathogens and UV irradiation [28]. In one study [29], poly(3-hydroxyalkanoate-co-3-hydroxyalkanoate) (PHAE) (an mcl-PHA) was coated with zosteric acid (nontoxic, antifouling agent) to come up with a PHA-based active food packaging. Exposing the coated PHAE to sewage sludge showed no signs of microbial growth [29]. In another work [30], Azotobacter chroococcum 23 was used to synthesize PHB in order to develop a PHA-based active food packaging. Antimicrobial agents, namely chemically synthesized benzoic acid and natural Silbiol were added to the PHB films and PHB-coated paper surface. The PHB films and PHB-coated paper surface exhibited no major antimicrobial activity against Gram-negative and Gram-positive bacterial strains [30]. For the purpose of preventing the antimicrobial agents such as benzoic acid from migrating into the food from the food packaging, Kwiecien et al. [31] produced an active food packaging system based on preservative-oligo (3-HB). Different concentrations of the antimicrobial agent vanillin (4-hydroxy-3-methoxybenzaldehyde) was added to the PHB solution [32]. The antimicrobial potential of the developed films was tested against a variety of bacterial strains and fungi. Both thermal and mechanical properties of the produced PHB films with and without vanillin were investigated and analyzed. The study concluded that in order to demonstrate antimicrobial activity, the minimum concentration of vanillin required is ≥50 μg/g PHB for fungi and ≥80 μg/g PHB for bacteria. Furthermore, the elongation at break for the PHB-vanillin exhibited a small increase when compared to that of PHB film. Nonetheless, both of the modulus of elasticity and tensile strength decreased. The rate of migration of vanillin at 37 °C into 50% ethanol and distilled water were 71.736 mg/mL and 65.54 mg/mL, respectively. This might be attributed to the higher temperature and faster migration of vanillin into 50% ethanol than distilled water [32].
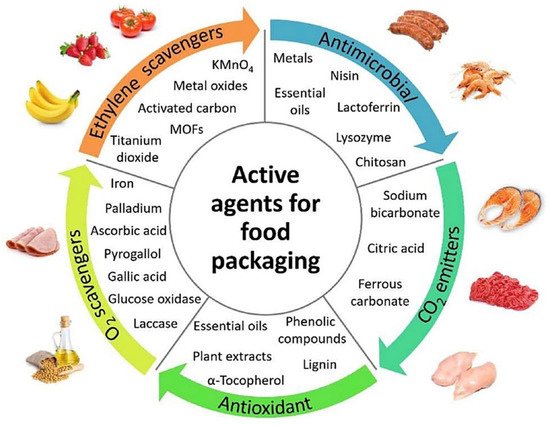
Figure 6. Bioactive agents for smart food packaging. Reprinted with permission from Elsevier, 2020 [28].

Figure 7. Active food packaging based on bio nanocomposites. Reprinted with permission from Elsevier, 2020 [28].
References
- Koyama, R.; Kuboki, T.; Ding, W.D.; Adhikary, K.B.; Chen, N.; Park, C.B. Extrusion foaming of cellulose fiber reinforced polylactic acid biocomposites. In Proceedings of the Annual Technical Conference—ANTEC, Boston, MA, USA, 1–5 May 2011.
- Cho, S.Y.; Park, H.H.; Yun, Y.S.; Jin, H.J. Influence of cellulose nanofibers on the morphology and physical properties of poly(lactic acid) foaming by supercritical carbon dioxide. Macromol. Res. 2013, 21, 529–533.
- Standau, T.; Zhao, C.; Castellón, S.M.; Bonten, C.; Altstädt, V. Chemical modification and foam processing of polylactide (PLA). Polymers 2019, 11, 306.
- Oluwabunmi, K.; D’Souza, N.A.; Zhao, W.; Choi, T.Y.; Theyson, T. Compostable, fully biobased foams using PLA and micro cellulose for zero energy buildings. Sci. Rep. 2020, 10, 1–20.
- Matuana, L.M.; Faruk, O. Effect of gas saturation conditions on the expansion ratio of microcellular poly (lactic acid)/wood-flour composites. Express Polym. Lett. 2010, 4, 621–631.
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300.
- Ding, W.D.; Kuo, P.Y.; Kuboki, T.; Park, C.B.; Sain, M. Foaming of cellulose fiber reinforced polylactic acid composites: The effect of cellulose fiber type. In Proceedings of the Annual Technical Conference—ANTEC, Cincinnati, OH, USA, 22–24 April 2013.
- Neagu, R.C.; Cuénoud, M.; Berthold, F.; Bourban, P.E.; Gamstedt, E.K.; Lindström, M.; Månson, J.A.E. The potential of wood fibers as reinforcement in cellular biopolymers. J. Cell. Plast. 2012, 48, 71–103.
- Matuana, L.M.; Diaz, C.A. Strategy to produce microcellular foamed poly(lactic acid)/wood-flour composites in a continuous extrusion process. Ind. Eng. Chem. Res. 2013, 52, 12032–12040.
- Bergeret, A.; Benezet, J.C. Natural fibre-reinforced biofoams. Int. J. Polym. Sci. 2011, 2011, 1–14.
- Pilla, S.; Kramschuster, A.; Lee, J.; Auer, G.K.; Gong, S.; Turng, L.S. Microcellular and solid polylactide-flax fiber composites. Compos. Interfaces 2009, 16, 869–890.
- Zafar, M.T.; Zarrinbakhsh, N.; Mohanty, A.K.; Misra, M.; Ghosh, A.K. Biocomposites based on poly(Lactic acid)/willow-fiber and their injection moulded microcellular foams. Express Polym. Lett. 2016, 10, 176–186.
- Noorani, R. 3D Printing Technology, Applications, and Selection, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018.
- Horvath, J. Mastering 3D Printing, 1st ed.; Apress: New York, NY, USA, 2014.
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742.
- Kikuchi, M.; Suetsugu, Y.; Tanaka, J.; Akao, M. Preparation and mechanical properties of calcium phosphate/copoly-L-lactide composites. J. Mater. Sci. Mater. Med. 1997, 8, 361–364.
- Niaza, K.V.; Senatov, F.S.; Kaloshkin, S.D.; Maksimkin, A.V.; Chukov, D.I. 3D-printed scaffolds based on PLA/HA nanocomposites for trabecular bone reconstruction. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2016; pp. 1–5.
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 1–21.
- Alam, F.; Verma, P.; Mohammad, W.; Teo, J.; Varadarajan, K.M.; Kumar, S. Architected poly(lactic acid)/poly(ε-caprolactone)/halloysite nanotube composite scaffolds enabled by 3D printing for biomedical applications. J. Mater. Sci. 2021, 56, 14070–14083.
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189.
- Ke, C.H.; Li, J.; Fang, K.Y.; Zhu, Q.L.; Zhu, J.; Yan, Q.; Wang, Y.Z. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym. Degrad. Stab. 2010, 95, 763–770.
- Tang, G.; Zhang, R.; Wang, X.; Wang, B.; Song, L.; Hu, Y.; Gong, X. Enhancement of flame retardant performance of bio-based polylactic acid composites with the incorporation of aluminum hypophosphite and expanded graphite. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 255–269.
- Wang, K.; Wang, J.; Zhao, D.; Zhai, W. Preparation of microcellular poly(lactic acid) composites foams with improved flame retardancy. J. Cell. Plast. 2017, 53, 45–63.
- Wang, J.; Ren, Q.; Zheng, W.; Zhai, W. Improved flame-retardant properties of poly(lactic acid) foams using starch as a natural charring agent. Ind. Eng. Chem. Res. 2014, 53, 1422–1430.
- Vadas, D.; Igricz, T.; Sarazin, J.; Bourbigot, S.; Marosi, G.; Bocz, K. Flame retardancy of microcellular poly(lactic acid) foams prepared by supercritical CO2-assisted extrusion. Polym. Degrad. Stab. 2018, 153, 100–108.
- Wu, N.; Yu, J.; Lang, W.; Ma, X.; Yang, Y. Flame retardancy and toughness of poly(lactic acid)/GNR/SiAHP composites. Polymers 2019, 11, 1129.
- Li, W.; Zhang, L.; Chai, W.; Yin, N.; Semple, K.; Li, L.; Zhang, W.; Dai, C. Enhancement of flame retardancy and mechanical properties of polylactic acid with a biodegradable fire-retardant filler system based on bamboo charcoal. Polymers 2021, 13, 2167.
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 1–12.
- Hany, R.; Böhlen, C.; Geiger, T.; Schmid, M.; Zinn, M. Toward non-toxic antifouling: Synthesis of hydroxy-cinnamic acid-, sulfate-, and zosteric acid-labeled poly. Biomacromolecules 2004, 5, 1452–1456.
- Gonta, S.; Savenkova, L.; Krallish, I.; Kirilova, E. Antimicrobial Activity of PHB Based Polymeric Compositions. Environ. Eng. Manag. J. 2012, 11, 99–104.
- Kwiecień, I.; Adamus, G.; Bartkowiak, A.; Kowalczuk, M. Synthesis and structural characterization at the molecular level of oligo(3-hydroxybutyrate) conjugates with antimicrobial agents designed for food packaging materials. Des. Monomers Polym. 2014, 17, 311–321.
- Xavier, J.R.; Babusha, S.T.; George, J.; Ramana, K.V. Material Properties and Antimicrobial Activity of Polyhydroxybutyrate (PHB) Films Incorporated with Vanillin. Appl. Biochem. Biotechnol. 2015, 176, 1498–1510.
More
