Acne vulgaris (AV) is a chronic skin disease of the pilosebaceous unit affecting both adolescents and adults. Its pathophysiology includes processes of inflammation, increased keratinization, sebum production, hormonal dysregulation, and bacterial Cutibacterium acnes proliferation. Common AV has been treated with antibiotics since the 1960s, but strain resistance has emerged and is of paramount concern. Macroalgae are known producers of substances with bioactive properties, including anti-viral, antibacterial, antioxidant, and anti-inflammatory properties, among several others. In particular, red algae are rich in bioactive compounds such as polysaccharides, phenolic compounds, lipids, sterols, alkaloids, and terpenoids, conferring them antioxidant, antimicrobial, and anti-inflammatory activities, among others. Thus, the exploration of compounds from marine resources can be an appealing approach to discover new treatment options against AV.
- antibacterial
- Cutibacterium acnes
- inflammation
- Rhodophyta
- seaweed extracts
1. Pathophysiological Targets for the Management of Acne Vulgaris
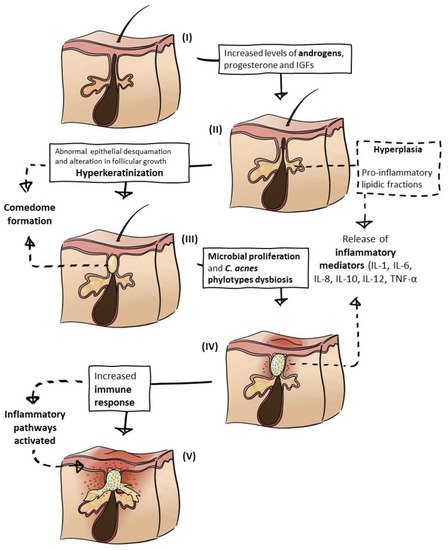
1.1. Hormonal Influence
1.2. Seborrhoea
The typical regions of the skin that are affected by acne are the face and upper torso, as they are densely populated by sebaceous glands [3][4]. Those glands are cutaneous appendages that secrete an oily substance (sebum) within hair follicles through sebaceous ducts [59][27]. Sebum is a mixture of lipids—namely a combination of wax esters, triglycerides, steroid esters, and squalene [60][28]—protecting the skin against friction due to natural lubrication; by acting as a barrier, it retains water molecules helping to keep the skin moist and healthy [42][19]. However, hyperseborrhea, excessive sebum production associated with overactive sebaceous glands, is a major etiopathogenetic factor directly associated with AV, because it contributes to all three mechanisms described below—comedogenesis, microbial proliferation, and inflammation—whereas the disease is not seen in the absence of sebum [4][7]. It is possible that not only the amount of sebum is a key factor in acne; the alteration of sebum composition may also have a determinant role. For instance, lipidic fractions of sebum are proinflammatory, contributing to the inflammatory cell and tissue process, ultimately resulting in the development of acne lesions [60,61][28][29]. For example, squalene peroxide induces inflammatory responses in keratinocytes by increasing IL-6 production and lipoxygenase (5-LOX) activation [62][30]. Moreover, modifications in the sebum ratio of saturated/unsaturated fatty acid are triggers of innate immunity response and follicular inflammation, being a main character in early inflammatory pathways of acne [47,60][24][28]. Additionally, changes in the oxidant/antioxidant ratio also represent a cause for acne’s initial development [32][9]. Further acne-related lipid-enzymes are liver X receptor-α (LXR-α) and cyclooxygenase 2 (COX-2), which regulate inflammation and lipid synthesis. LXR-α is expressed in sebaceous glands, sweat glands, and hair follicles, and controls the transcription of the genes involved in fatty acid and lipid synthesis. During inflammatory events, COX2 release is stimulated by cytokines that were activated by NF-κB [63][31].1.3. Comedogenesis
Increased androgen production, the accumulation of sebum, and the adhesiveness of keratinocytes lead to follicle blockage and the occlusion of the pilosebaceous ducts, resulting in the formation of microcomedones that are the primary type of acne lesions [7,64][32][33]. These hard structures, microcomedones, are formed within the hair follicle, more precisely in the infundibulum, and are connected with the sebaceous gland via a keratinized duct. The set of the hair follicle and sebaceous gland constitutes the pilosebaceous unit [59][27]. As a pleomorphic disorder of the pilosebaceous unit, acne microcomedones evolve into comedones owing to sebum and keratin accumulation accompanied by follicle expansion [58][34], and these can be closed (white heads) or open (blackheads), but they may progress to inflammatory lesions, such as pustules, papules, cysts, and nodules [30][5], eventually leaving scars [6][8]. Keratinocyte IL-1 secretion is another triggering step in the comedogenesis process, mostly as a consequence of C. acnes-mediated Toll-like receptor (TLR) activation. IL-1 also contributes to sebocytes hypercornification. Comedone formation is a result of a combination of some factors, such as the response to androgen production; sebum alteration, production, and oxidation; C. acnes proliferation, and recruitment of cytokines to the pilosebaceous unit [7][32].1.4. Inflammatory Response
The AV pathophysiological cascade ends with the inflammatory process, but, at the same time, it also follows the whole process from the beginning, by activating the immune system and/or by the release of molecules that activate inflammatory pathways [71][35]. There are many inflammatory mediators involved, as mentioned before, e.g., proinflammatory lipids (at the hyperseborrhea process), cytokines (mostly involved by recruitment of C. acnes), and chemokines (stimulated by bacterial antigens). There is also the role of proinflammatory cathelicidins, peptides from macrophage lysosomes, and members of the immune system with immunomodulatory and antimicrobial functions [7][32]. It seems that the main factor influencing the inflammatory process is the activation of the immune system by C. acnes, when the bacterium overpopulates sebocytes, through TLRs and nod-like receptors (NLRs) that segregate IL-1 and other cytokines, activating inflammatory pathways [7][32]. Furthermore, the keratinocyte stimulated-C. acnes production of reactive oxygen species (ROS) induces nitric-oxide production in macrophages, contributing to the magnification of the inflammatory response [69][36].2. Seaweed Extracts and Compounds to Address AV Disease
Numerous studies have identified red seaweeds as significant producers of valuable metabolites with antibacterial and anti-inflammatory activities, thus representing a potential resource in the battle against AV, a chronic disease of which the management involves the mitigation of these symptoms.
2.1. Antibacterials from Red Macroalgae
The study by Choi and co-workers [83][37] employed methanol solid–liquid extractions from S. latiuscula, resulting in an extract capable of inhibiting C. acnes growth. This seaweed is known to have high amounts of bromophenols, which can explain the antibacterial effect, because these compounds are known to be toxic to some bacteria [83][37]. The work of Barreto and Meyer (2006) aimed to isolate lanosol ethyl ether from O. serrata, which is a brominated phenol and a halogenated metabolite, showing high bacteriostatic and mild bactericidal activity [23][38]. Organobromine compounds are naturally produced by seaweeds for chemical defense and are usually found in Rhodophyta. Besides lanosol, examples include acetogenins (brominated nonterpenoid metabolites), which are mainly found in the genus Laurencia [84][39]; bromoform from Asparagopsis taxiformis [85][40]; brominated monoterpenes, also in the genus Laurencia [86][41], but also in Plocamium cartilagineum [84][39]; and indoles from Rhodophyllis membranacea [84][39]. These bromine compounds confer several bioactivities, including antimicrobial, antioxidant, anticancerogenic, and anti-diabetic activities [87][42], emphasizing the high value of red seaweed-derived compounds. Another example showing the antibacterial activity conferred by organobromine compounds is the study by [88][43], which used Asparagopsis armata supercritical CO2 extractions to find an inhibition halo of 23 mm (at 10 mg·mL−1) in contact with a C. acnes culture. Such results seem to be in accordance with a variety of cosmeceutical products which recently became available in the market that incorporate A. armata extracts. For instance, ASPAR’AGE™, formulated by SEPPIC (La Garenne-Colombes, France), promises a lotion with an extract containing MAAs that reduce the visible effects of age, and Asparcid P®, from Exsymol (Monaco-Ville, Monaco), claims to possess cytostimulating action and antimicrobial activity.
2.2. Extracts and Compounds from Red Macroalgae Targeting Other Mechanisms of AV
The literature search on anti-sebum compounds or extracts from Rhodophyta, even when it comes to macroalgae in general, revealed a complete absence of published studies. Despite this fact, SEPPIC (La Garenne-Colombes, France), a cosmetic company, own red seaweed-incorporating products in the market claiming to possess anti-sebum properties. The incorporation of macroalgal extracts in the skincare line product WESOURCE (SEPPIC) revealed an anti-sebum effect (34% reduction after 56 days), observed when oily skinned volunteers were tested using CONTACTICEL™ (SEPPIC, La Garenne-Colombes, France) [199][44], a skin-care product that contained extracts from Acrochaetium moniliforme, a red seaweed product under the patent WO2016162648A1. Furthermore, similar results were obtained with a brown seaweed, revealing in vivo sebum regulation—29.3% lower sebum in 23 days—providing visible mattifying effects on volunteers subjected to Laminaria saccharina extract [199][44]. Because both cosmetic products are effective in the reduction of facial sebum, using active ingredients obtained from two macroalgae, despite no scientific publications being found, at least some red and brown seaweeds may be considered to have anti-sebum properties in vivo. Hyperlipidaemia, a condition characterized by high levels of lipids in the blood, e.g., cholesterol, was associated with patients exhibiting acne. In accordance with this, high levels of triglycerides (TG), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) were found in the blood of both male and female subjects [200,201][45][46]. A study by Liu et al. (2017) showed the improvement of carbohydrate and lipid metabolism in rats which were hyperlipidaemia-induced by high fructose (HF) intake, when fed with a Gelidium amansii-supplemented diet. Gelidium amansii supplementation resulted in the decrease of glucose, leptin, insulin, and TNF-α blood levels [202][47]. Moreover, it also reduced the accumulation of hepatic lipids, namely TG and the total cholesterol (TC) content, while increasing the excretion of bile acid and faecal lipids [202][47]. Further studies are required to prove Rhodophyta’s anti-hyperlipidaemia properties, but evidence already shows the potential of using this biomass to reduce the lipid levels in acne patients.References
- Thiboutot, D.; Dréno, B.; Sanders, V.; Rueda, M.J.; Gollnick, H. Changes in the management of acne: 2009–2019. J. Am. Acad. Dermatol. 2020, 82, 1268–1269.
- Kurokawa, I.; Nakase, K. Recent advances in understanding and managing acne. F1000Research 2020, 9, 792.
- Zouboulis, C.C. Endocrinology and immunology of acne: Two sides of the same coin. Exp. Dermatol. 2020, 29, 840–859.
- Tan, J.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172, 3–12.
- Dawson, A.L.; Dellavalle, R. Acne vulgaris. BMJ 2013, 346, f2634.
- Araviiskaia, E.; Dréno, B. The role of topical dermocosmetics in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 926–935.
- Aydemir, E.H. Acne vulgaris. Türk Pediatri Arşivi 2017, 49, 13–16.
- Arora, M.K.; Yadav, A.; Saini, V. Role of hormones in acne vulgaris. Clin. Biochem. 2011, 44, 1035–1040.
- Bhat, Y.; Latief, I.; Hassan, I. Update on etiopathogenesis and treatment of Acne. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 298–306.
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.-P.; Leccia, M.; Del Giudice, P.; Dreno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278.
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes(Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14.
- Gollnick, H.P.M. From new findings in acne pathogenesis to new approaches in treatment. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1–7.
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R. Acne Vulgaris: Pathogenesis, Treatment, and Needs Assessment. Dermatol. Clin. 2012, 30, 99–106.
- Li, X.; He, C.; Chen, Z.; Zhou, C.; Gan, Y.; Jia, Y. A review of the role of sebum in the mechanism of acne pathogenesis. J. Cosmet. Dermatol. 2017, 16, 168–173.
- Tuchayi, S.M.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.P.D. Acne vulgaris. Nat. Rev. Dis. Prim. 2015, 1, 1–20.
- Cappel, M. Correlation Between Serum Levels of Insulin-like Growth Factor 1, Dehydroepiandrosterone Sulfate, and Dihydrotestosterone and Acne Lesion Counts in Adult Women. Arch. Dermatol. 2005, 141, 333–338.
- Rocha, M.A.; Bagatin, E. Adult-onset acne: Prevalence, impact, and management challenges. Clin. Cosmet. Investig. Dermatol. 2018, 11, 59–69.
- Belgorosky, A.; Guercio, G.; Pepe, C.; Saraco, N.; Rivarola, M. Genetic and Clinical Spectrum of Aromatase Deficiency in Infancy, Childhood and Adolescence. Horm. Res. Paediatr. 2009, 72, 321–330.
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C.C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermato. Endocrinol. 2011, 3, 41–49.
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and Structural Biology of Androgen Receptor. Chem. Rev. 2005, 105, 3352–3370.
- Balachandrudu, B.; Niveditadevi, V.; Rani, T.P. Hormonal Pathogenesis of Acne–Simplified. Int. J. Sci. Study 2015, 3, 183–185.
- Hu, T.; Wei, Z.; Ju, Q.; Chen, W. Sex hormones and acne: State of the art. J. der Dtsch. Dermatol. Ges. 2021, 19, 509–515.
- Szöllősi, A.G.; Oláh, A.; Bíró, T.; Tóth, B.I. Recent advances in the endocrinology of the sebaceous gland. Dermato-Endocrinology 2017, 9, e1361576.
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40.
- Bagatin, E.; De Freitas, T.H.P.; Rivitti-Machado, M.C.; Ribeiro, B.M.; Nunes, S.; Da Rocha, M.A.D. Adult female acne: A guide to clinical practice. An. Bras. de Dermatol. 2019, 94, 62–75.
- Cole, T.; Ahmed, M.; Preece, M.; Hindmarsh, P.; Dunger, D. The relationship between Insulin-like Growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin. Endocrinol. 2015, 82, 862–869.
- Clayton, R.; Göbel, K.; Niessen, C.; Paus, R.; Van Steensel, M.; Lim, X. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br. J. Dermatol. 2019, 181, 677–690.
- Chen, F.; Hu, X.; Dong, K. Consistency changes of potential lipid markers in acne patients of different ages and their role in acne pathogenesis. J. Cosmet. Dermatol. 2021, 20, 2031–2035.
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334.
- Zouboulis, C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 527–532.
- Bakry, O.A.; El Farargy, S.M.; Kady, N.N.E.D.E.; Abu Dawy, H.F. Immunohistochemical Expression of Cyclo-oxygenase 2 and Liver X Receptor-α in Acne Vulgaris. J. Clin. Diagn. Res. 2017, 11, WC01–WC07.
- Greydanus, D.E.; Azmeh, R.; Cabral, M.D.; Dickson, C.A.; Patel, D.R. Acne in the first three decades of life: An update of a disorder with profound implications for all decades of life. Dis. Mon. 2021, 67, 101103.
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349.
- Tanghetti, E.A. The Role of Inflammation in the Pathology of Acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27–35.
- Harvey, A.; Huynh, T.T. Inflammation and acne: Putting the pieces together. J. drugs Dermatol. JDD 2014, 13, 459–463.
- Mayslich, C.; Grange, P.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303.
- Choi, J.-S.; Bae, H.-J.; Kim, S.-J.; Choi, I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318.
- Barreto, M.; Meyer, J. Isolation and antimicrobial activity of a lanosol derivative from Osmundaria serrata (Rhodophyta) and a visual exploration of its biofilm covering. S. Afr. J. Bot. 2006, 72, 521–528.
- Gribble, G.W. The natural production of organobromine compounds. Environ. Sci. Pollut. Res. 2000, 7, 37–49.
- Burreson, B.J.; Moore, R.E.; Roller, P.P. Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta). J. Agric. Food Chem. 1976, 24, 856–861.
- Suzuki, M.; Takahashi, Y.; Nakano, S.; Abe, T.; Masuda, M.; Ohnishi, T.; Noya, Y.; Seki, K.-I. An experimental approach to study the biosynthesis of brominated metabolites by the red algal genus Laurencia. Phytochemistry 2009, 70, 1410–1415.
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292.
- Lee, K.W.; Heo, S.H.; Lee, J.; Park, S.I.; Kim, M.; Shin, M.S. Antimicrobial, Antioxidative, Elastase and Tyrosinase Inhibitory Effect of Supercritical and Hydrothermal Halopteris scoparia Extract. Turk. J. Comput. Math. Educ. (TURCOMAT) 2021, 12, 407–413.
- Gillon, L.-A. WESOURCE—Active Science to Empower Beauty. From the Sea to the Skin—How Algae Adaptations Benefit Skin & Hair? Available online: https://www.flickr.com/photos/fotoosvanrobin/3644416895 (accessed on 27 September 2021).
- Sobhan, M.; Rabiei, M.A.S.; Amerifar, M. Correlation Between Lipid Profile and Acne Vulgaris. Clin. Cosmet. Investig. Dermatol. 2020, 13, 67–71.
- Jiang, H.; Li, C.Y.; Zhou, L.; Lu, B.; Lin, Y.; Huang, X.; Wei, B.; Wang, Q.; Wang, L.; Lu, J.; et al. Acne patients frequently associated with abnormal plasma lipid profile. J. Dermatol. 2015, 42, 296–299.
- Liu, H.-C.; Chang, C.-J.; Yang, T.-H.; Chiang, M.-T. Long-term feeding of red algae (Gelidium amansii) ameliorates glucose and lipid metabolism in a high fructose diet-impaired glucose tolerance rat model. J. Food Drug Anal. 2016, 25, 543–549.
