Chaperone-usher fimbrial adhesins are powerful weapons against the uropathogens that allow the establishment of urinary tract infections (UTIs). As the antibiotic therapeutic strategy has become less effective in the treatment of uropathogen-related UTIs, the anti-adhesive molecules active against fimbrial adhesins, key determinants of urovirulence, are attractive alternatives. The best-characterized bacterial adhesin is FimH, produced by uropathogenic Escherichia coli (UPEC). Hence, a number of high-affinity mono- and polyvalent mannose-based FimH antagonists, characterized by different bioavailabilities, have been reported. Given that antagonist affinities are firmly associated with the functional heterogeneities of different FimH variants, several FimH inhibitors have been developed using ligand-drug discovery strategies to generate high-affinity molecules for successful anti-adhesion therapy. As clinical trials have shown d-mannose’s efficacy in UTIs prevention, it is supposed that mannosides could be a first-in-class strategy not only for UTIs, but also to combat other Gram-negative bacterial infections.
- FimH
- adhesins
- uropathogenic Escherichia coli
- uropathogenic Klebsiella pneumoniae
- uropathogenic Proteus mirabilis
- urinary tract infection
- antagonists
- mannose-binding lectin
- affinity
1. Introduction
2. FimH is a Highly Adapted Virulence Factor
Among the CU pili from uropathogenic members belonging to Enterobacteriaceae, one of the best-characterized is type 1 pili. These pili are expressed by 80% and 90% of UPKP and UPEC strains, respectively [5,47,48,49][5][18][19][20]. It has been reported that more than 95% of all E. coli isolates express type 1 fimbriae [50,51,52][21][22][23]. The type 1 pilus is 2 μm in length and 10 nm in width, and is highly represented in the bacterial surface (100–500 pili per cell) [5,47,48,49][5][18][19][20]. This pilus is defined as mannose-sensitive, because it is able to interact with the mannosylated receptors expressed by epithelial cells, particularly urothelial cells [12,19,48,53,54][12][24][19][25][26]. This specific function relies on the expression of the adhesin FimH located at the tip of the type 1 pilus. Type 1 pili of K. pneumoniae and E. coli are highly homologous in uropathogenic strains. However, the slight sequence variations between the FimH from E. coli and K. pneumoniae result in huge differences in the ability to colonize the urinary tract, FimH from UPEC being much more efficient in adhering to the mannosylated structure; for these reasons, it was chosen as the model for the bacteria–urothelium interaction [55][27]. Proper functioning of urothelium depends on the precise assemblage of highly specialized glycoproteins called uroplakins (UPs), the end products and differentiation markers of urothelial cells. On the apical surface, four major UPs are expressed by the umbrella cells lining the bladder, forming hexagonal plaques characterized by six tetramers linked by two heterodimers, UPIa/II and Ib/IIIa. UPs can be synthesized in several glycoforms; UPIa contains high-mannose N-glycans, and UPIb and IIIa carry complex N-glycans, whereas mature UPII lacks sugar moieties [56,57][28][29]. FimH binds to high-mannosylated UPIa, thereby ensuring a stable bacterial adhesion to the tissue. It is noteworthy that FimH is also responsible for biofilm formation, proliferation, and invasion of and internalization into eukaryotic cells, mediating the formation of intracellular bacterial communities (IBCs) [8,54,58][8][26][30]. Moreover, FimH is also able to interact with the Tamm–Horsfall soluble proteins, which are secreted by kidney cells, within the urine to exert a protective role against FimH adhesion [53,59][25][31]. Finally, CD48, types I and IV collagens, fibronectins and laminins are other receptors that can be bound by the UPEC FimH [60][32]. Due to the multiplicity of ligands and functions, type 1 pili armed with FimH represent a pivotal virulence factor within UPEC [5,8,19,30,61][5][8][24][33][34]. The whole FimH adhesin is composed of 279 amino acids. The N-terminal domain (NTD) carries a lectin domain (FimHLD) encompassing the carbohydrate-binding domain (CBD), while the C-terminal domain (CTD) bears a pilin domain (FimHPD) (Figure 1) [19,54,62][24][26][35]. The interaction between these two domains, FimHLD and FimHPD, determines the conformational state of the FimH adhesin, thereby influencing the level of affinity of FimH with the related molecule/receptor/ligand [13,54][13][26]. The conformation of FimH is highly dynamic, and interdomain interactions can be influenced by different factors, including the shear stress. Normally, FimH is in the low affinity conformation, also known as T-state; however, it switches to the high affinity structure in the presence of shear forces (R-state). The mechanism by which FimH binds to the mannosylated uroplakins is known as the catch and bond mechanism, which enables bacteria to establish long-lived interactions with host cells [13,54][13][26].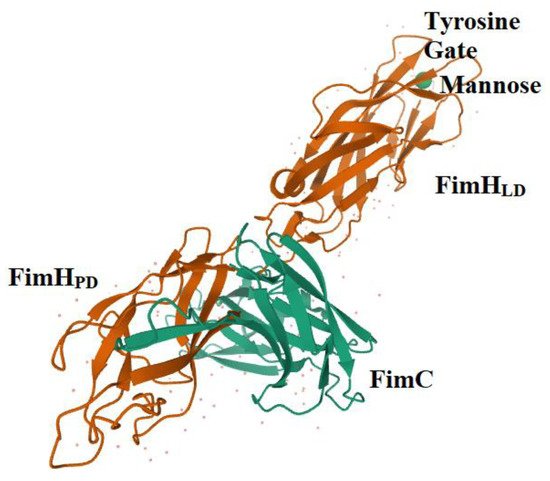
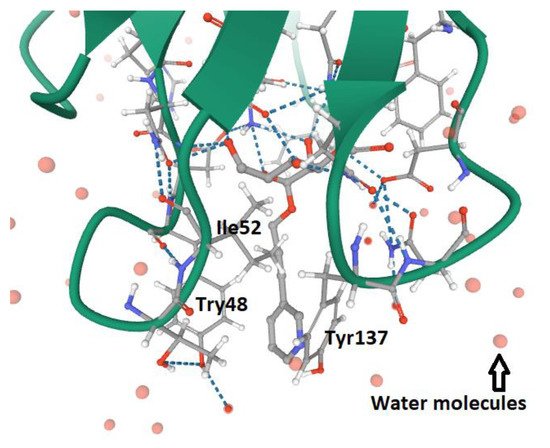
3. FimH and Glycomimetics
4. FimH Antagonists, Biochemical Characteristics and Bioavailability
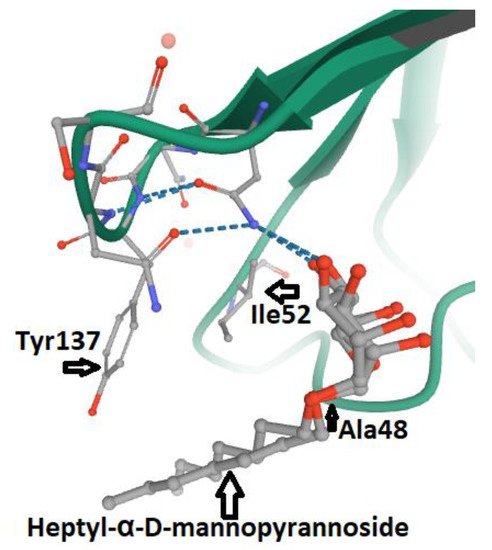
According to the type of interaction with the d-mannosylated molecules, FimH adhesin changes its conformational structure, leading to different binding affinities. As outlined above, the low affinity (T-state) conformational structure of FimH, in which the LD and PD domains are in strict contact, occurs in the absence of shear stress. Vice versa, the high affinity conformation (R-state), in which FimHLD and FimPD are separated, represents the shear stress-induced allosteric regulation of its mannose-binding affinity, resulting in the strong attachment of FimHLD to the host urothelial cell receptors [54][26]. Thus, the balance between R- and T-states regulates the capability of the bacteria to colonize the urothelial niche or to spread the infection. At the molecular level, it is known that the interactions between α-d-mannose molecules (and related derivatives) and MBP in FimHLD occur in the presence of water, because water molecules support the hydrogen bonds between the hydroxyl groups of α-d-mannose molecules and the amino acid residues within MBP. Moreover, the presence of water drives the proper binding of α-anomer molecules to the MBP of FimH, increasing the affinity of the α-anomeric configuration of mannose and its derivatives with MBP [19,62,95,96][24][35][57][58]. Biochemical analyses of the interaction between FimHLD and α-d-mannose revealed that mannosides with an apolar (hydrophobic) substituent are able to mimic the interactions of high-mannose glycans with the MBD of FimH [82][56]. For this reason, n-hexyl- and n-heptyl-modified mannosides (i.e., MeMan) have a significant high affinity towards FimH [19,97][24][59]. This hydrophobic portion of aglycone interacts with the tyrosine gate through aromatic stacking (non-covalent interaction between aromatic rings) and Van der Waals bonds [77,97,98,99][51][59][60][61]. Moreover, it was shown that glycomimetics with inhibition constants in the range of 1–20 nM can be obtained by combining the α-anomeric configurations of d-mannose [96,100][58][62]. Hence, Wellens et al. generated a set of α-d-mannosides carrying alkyl and aryl hydrophobic moieties. The determination of the crystal structure of FimHLD with the eight synthesized inhibitors, together with the analyses of their thermodynamic parameters, demonstrated that the presence of alkyl and aryl groups in the aglycone can induce the increased dynamics in the tyrosine gate responsible for the proper orientation of the interacting mannosides. This dynamic behavior of the tyrosine gate could contribute to FimH’s ability to deal with less compatible high-mannose structures, while still making bacterial adhesion plausible [64][37]. Moreover, aromatic aglycone compounds mediate several interactions within the tyrosine gate in its hydrophobic space, increasing the affinity of the antagonist to the MBP of FimHLD [80,96][54][58]. An increase in the length of alkyl chains results in the higher affinity of the molecule with the FimHLD and, in particular, with the tyrosine gate area, showing that the affinity of the alkyl group with FimH adhesin is 100-fold greater than that exhibited by mannose [19,25,89][24][63][64]. It has been shown that O- and C-linked α-d-mannosides with hydrophobic and aryl substituents are potent E. coli FimH antagonists, having an affinity in the same range as that of nanomolar [101][65]. Indeed, the conformation and lipophilicity of aglycone moieties, their position with respect to the core sugar structure and the type of chemical group determine the RIP of antagonist molecules [61,80][34][54]. Para-substituted biphenyl derivatives were shown to be particularly appealing, owing to their numerous favorable binding interactions within the tyrosine gate. Thus, the structural and functional analyses of a series of O-, C-, and S-linked mannoside derivatives, incorporating the 1,1′-biphenyl pharmacophore and diverse aglycone atoms, demonstrated the suitability of these antagonists, establishing the possibility of further exploring these chemically modified mannosides [101,102][65][66]. Furthermore, it was shown that the biphenyl group linked to mannosides can be efficiently absorbed if orally administered [54,88][26][67]. Indeed, these mannosides show increased metabolic stability, bioavailability and intestinal permeability in in vivo pharmacokinetic studies, thereby recommending them for preclinical evaluation [83][68]. In addition, the reabsorption of biphenyl groups by renal tubuli results in stable and regular excretion into urine, leading to their high availability in the site of infection [54,88,103][26][67][69]. It was also demonstrated that 3′-chloro-4′-(α-d-mannopyranosyloxy) biphenyl-4-carbonitriler (Figure 4), synthesized using the bioisostere approach, is a highly effective FimH antagonist, also presenting optimal pharmacokinetic characteristics, such as proper solubility, low toxicity, intestinal permeability and renal excretion in mouse models [47][18]. Moreover, its oral application reduced the bacterial load in the bladder by almost 1000-fold 3 h after infection, highlighting its therapeutic potential [47][18].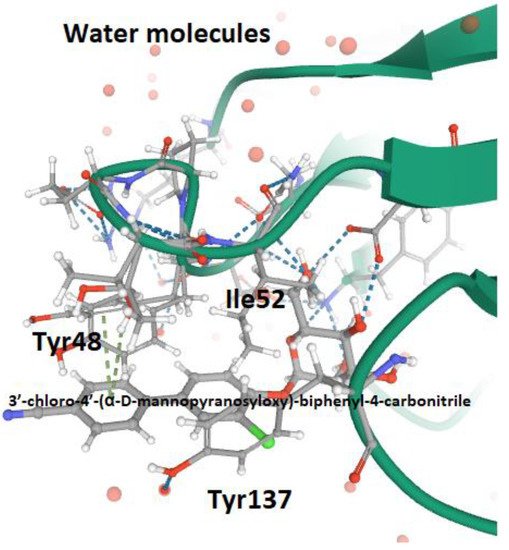
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284.
- Behzadi, P.; Behzadi, E.; Pawlak-Adamska, E.A. Urinary tract infections (UTIs) or genital tract infections (GTIs)? It’s the diagnostics that count. GMS Hyg. Infect. Control 2019, 14.
- Chockalingam, A.; Stewart, S.; Xu, L.; Gandhi, A.; Matta, M.K.; Patel, V.; Sacks, L.; Rouse, R. Evaluation of immunocompetent urinary tract infected Balb/C mouse model for the study of antibiotic resistance development using Escherichia Coli CFT073 infection. Antibiotics 2019, 8, 170.
- Issakhanian, L.; Behzadi, P. Antimicrobial agents and urinary tract infections. Curr. Pharm. Des. 2019, 25, 1409–1423.
- Behzadi, P. Classical chaperone-usher (CU) adhesive fimbriome: Uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs). Folia Microbiol. (Praha) 2020, 65, 45–65.
- Hozzari, A.; Behzadi, P.; Kerishchi Khiabani, P.; Sholeh, M.; Sabokroo, N. Clinical cases, drug resistance, and virulence genes profiling in Uropathogenic Escherichia coli. J. Appl. Genet. 2020, 61, 265–273.
- Momtaz, H.; Karimian, A.; Madani, M.; Safarpoor Dehkordi, F.; Ranjbar, R.; Sarshar, M.; Souod, N. Uropathogenic Escherichia coli in Iran: Serogroup distributions, virulence factors and antimicrobial resistance properties. Ann. Clin. Microbiol. Antimicrob. 2013, 12.
- Jahandeh, N.; Ranjbar, R.; Behzadi, P.; Behzadi, E. Uropathogenic Escherichia coli virulence genes: Invaluable approaches for designing DNA microarray probes. Cent. Eur. J. Urol. 2015, 68, 452–458.
- Behzadi, P.; Najafi, A.; Behzadi, E.; Ranjbar, R. Microarray long oligo probe designing for Escherichia coli: An in-silico DNA marker extraction. Cent. Eur. J. Urol. 2016, 69, 105–111.
- Scribano, D.; Sarshar, M.; Prezioso, C.; Lucarelli, M.; Angeloni, A.; Zagaglia, C.; Palamara, A.T.; Ambrosi, C. D-Mannose Treatment neither Affects Uropathogenic Escherichia coli Properties nor induces stable fimh modifications. Molecules. 2020, 25, 316.
- Umpiérrez, A.; Scavone, P.; Romanin, D.; Marqués, J.M.; Chabalgoity, J.A.; Rumbo, M.; Zunino, P. Innate immune responses to proteus mirabilis flagellin in the urinary tract. Microbes Infect. 2013, 15, 688–696.
- Behzadi, E.; Behzadi, P. The role of toll-like receptors (TLRs) in urinary tract infections (UTIs). Cent. Eur. J. Urol. 2016, 69, 404–410.
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566.
- Behzadi, P.; Urbán, E.; Matuz, M.; Benkő, R.; Gajdács, M. The role of gram-negative bacteria in urinary tract infections: Current concepts and therapeutic options. Adv. Exp. Med. Biol. 2020, 10.
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and urinary tract infections. Microbiol. Spectr. 2015, 3.
- Wyres, K.L.; Lam, M.; Holt, K.E. Population genomics of klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359.
- Psonis, J.J.; Thanassi, D.G. Therapeutic approaches targeting the assembly and function of chaperone-usher pili. EcoSal Plus 2019, 8.
- Kleeb, S.; Pang, L.; Mayer, K.; Eris, D.; Sigl, A.; Preston, R.C.; Zihlmann, P.; Sharpe, T.; Jakob, R.P.; Abgottspon, D.; et al. FimH antagonists: Bioisosteres to improve the in vitro and in vivo PK/PD profile. J. Med. Chem. 2015, 58, 2221–2239.
- Hartmann, M.; Papavlassopoulos, H.; Chandrasekaran, V.; Grabosch, C.; Beiroth, F.; Lindhorst, T.K.; Röhl, C. Inhibition of bacterial adhesion to live human cells: Activity and cytotoxicity of synthetic mannosides. FEBS Lett. 2012, 586, 1459–1465.
- Stahlhut, S.G.; Struve, C.; Krogfelt, K.A. Klebsiella pneumoniae type 3 fimbriae agglutinate yeast in a mannose-resistant manner. J. Med. Microbiol. 2012, 61, 317–322.
- Sokurenko, E.V.; Chesnokova, V.; Dykhuizen, D.E.; Ofek, I.; Wu, X.R.; Krogfelt, K.A.; Struve, C.; Schembri, M.A.; Hasty, D.L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc. Nat.l Acad. Sci. USA 1998, 95, 8922–8926.
- Sarshar, M.; Scribano, D.; Marazzato, M.; Ambrosi, C.; Aprea, M.R.; Aleandri, M.; Pronio, A.; Longhi, C.; Nicoletti, M.; Zagaglia, C.; et al. Genetic diversity, phylogroup distribution and virulence gene profile of pks positive Escherichia coli colonizing human intestinal polyps. Microb. Pathog. 2017, 112, 274–278.
- Ambrosi, C.; Sarshar, M.; Aprea, M.R.; Pompilio, A.; Di Bonaventura, G.; Strati, F.; Pronio, A.; Nicoletti, M.; Zagaglia, C.; Palamara, A.T.; et al. Colonic adenoma-associated Escherichia coli express specific phenotypes. Microbes Infect. 2019, 21, 305–312.
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational design strategies for FimH antagonists: New drugs on the horizon for urinary tract infection and Crohn’s disease. Expert Opin. Drug Discov. 2017, 12, 711–731.
- Rafsanjany, N.; Senker, J.; Brandt, S.; Dobrindt, U.; Hensel, A. In vivo consumption of cranberry exerts ex vivo antiadhesive activity against FimH-Dominated uropathogenic Escherichia coli: A combined in vivo, ex vivo, and in vitro study of an extract from vaccinium macrocarpon. J. Agric. Food Chem. 2015, 63, 8804–8818.
- Mayer, K.; Eris, D.; Schwardt, O.; Sager, C.P.; Rabbani, S.; Kleeb, S.; Ernst, B. Urinary tract infection: Which conformation of the bacterial lectin FimH is therapeutically relevant? J. Med. Chem. 2017, 60, 5646–5662.
- Rosen, D.A.; Pinkner, J.S.; Walker, J.N.; Elam, J.S.; Jones, J.M.; Hultgren, S.J. Molecular variations in Klebsiella pneumoniae and Escherichia coli FimH affect function and pathogenesis in the urinary tract. Infect. Immun. 2008, 76, 3346–3356.
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J. Cell Sci. 2001, 114, 4095–4103.
- Kątnik-Prastowska, I.; Lis, J.; Matejuk, A. glycosylation of uroplakins. Implications for bladder physiopathology. Glycoconj. J. 2014, 31, 623–636.
- Lewis, A.J.; Richards, A.C.; Mulvey, M.A. Invasion of host cells and tissues by uropathogenic bacteria. Microbiol. Spectr. 2016, 4.
- Bates, J.M.; Raffi, H.M.; Prasadan, K.; Mascarenhas, R.; Laszik, Z.; Maeda, N.; Hultgren, S.J.; Kumar, S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: Rapid communication. Kidney Int. 2004, 65, 791–797.
- Eto, D.S.; Jones, T.A.; Sundsbak, J.L.; Mulvey, M.A. Integrin-Mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007, 3.
- Zalewska-Piątek, B.M.; Piątek, R.J. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim. Pol. 2019, 66, 129–138.
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Effective anti-adhesives of uropathogenic Escherichia coli. Acta Pharm. 2018, 68, 1–18.
- Hung, C.S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002, 44, 903–915.
- Mydock-McGrane, L.K.; Cusumano, Z.T.; Janetka, J.W. Mannose-Derived FimH antagonists: A promising anti-virulence therapeutic strategy for urinary tract infections and Crohn’s disease. Expert Opin. Ther. Pat. 2016, 26, 175–197.
- Wellens, A.; Lahmann, M.; Touaibia, M.; Vaucher, J.; Oscarson, S.; Roy, R.; Remaut, H.; Bouckaert, J. The tyrosine gate as a potential entropic lever in the receptor-binding site of the bacterial adhesin FimH. Biochemistry 2012, 51, 4790–4799.
- Rabbani, S.; Krammer, E.M.; Roos, G.; Zalewski, A.; Preston, R.; Eid, S.; Zihlmann, P.; Prévost, M.; Lensink, M.F.; Thompson, A.; et al. Mutation of Tyr137 of the universal Escherichia coli fimbrial adhesin FimH relaxes the tyrosine gate prior to mannose binding. IUCr J. 2017, 4, 7–23.
- Chen, S.L.; Hung, C.S.; Pinkner, J.S.; Walker, J.N.; Cusumano, C.K.; Li, Z.; Bouckaert, J.; Gordon, J.I.; Hultgren, S.J. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. USA 2009, 106, 22439–22444.
- Duguid, J.P.; Gillies, R.R. Fimbriæ and adhesive properties in dysentery bacilli. J. Pathol. Bacteriol. 1957, 74.
- Ofek, I.; Mirelman, D.; Sharon, N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature 1977, 265, 623–625.
- Firon, N.; Ofek, I.; Sharon, N. Interaction of mannose-containing oligosaccharides with the fimbrial lectin of Escherichia coli. Biochem. Biophys. Res. Commun. 1982, 105, 1426–1432.
- Firon, N.; Ofek, I.; Sharon, N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr. Res. 1983, 120, 235–249.
- Neeser, J.R.; Koellreutter, B.; Wuersch, P. Oligomannoside-type glycopeptides inhibiting adhesion of Escherichia coli strains mediated by type 1 pili: Preparation of potent inhibitors from plant glycoproteins. Infect. Immun. 1986, 52, 428–436.
- Koliwer-Brandl, H.; Siegert, N.; Umus, K.; Kelm, A.; Tolkach, A.; Kulozik, U.; Kuballa, J.; Cartellieri, S.; Kelm, S. Lectin inhibition assays for the analysis of bioactive milk sialoglycoconjugates. Int. Dairy J. 2011, 21, 413–420.
- Chalopin, T.; Brissonnet, Y.; Sivignon, A.; Deniaud, D.; Cremet, L.; Barnich, N.; Bouckaert, J.; Gouin, S.G. Inhibition profiles of mono- and polyvalent FimH antagonists against 10 different Escherichia coli strains. Org. Biomol. Chem. 2015, 13, 11369–11375.
- Mydock-McGrane, L.; Cusumano, Z.; Han, Z.; Binkley, J.; Kostakioti, M.; Hannan, T.; Pinkner, J.S.; Klein, R.; Kalas, V.; Crowley, J.; et al. Antivirulence c-mannosides as antibiotic-sparing, oral therapeutics for urinary tract infections. J. Med. Chem. 2016, 59, 9390–9408.
- Sattin, S.; Bernardi, A. Glycoconjugates and glycomimetics as microbial anti-adhesives. Trends Biotechnol. 2016, 34, 483–495.
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677.
- Firon, N.; Ashkenazi, S.; Mirelman, D.; Ofek, I.; Sharon, N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 1987, 55, 472–476.
- Vanwetswinkel, S.; Volkov, A.N.; Sterckx, Y.G.; Garcia-Pino, A.; Buts, L.; Vranken, W.F.; Bouckaert, J.; Roy, R.; Wyns, L.; van Nuland, N.A. Study of the structural and dynamic effects in the FimH adhesin upon α-d-heptyl mannose binding. J. Med. Chem. 2014, 57, 1416–1427.
- Chabre, Y.M.; Roy, R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013, 42, 4657–4708.
- Lee, Y.C.; Lee, R.T. Carbohydrate-protein interactions: Basis of glycobiology. Acc. Chem. Res. 1995, 28, 321–327.
- Hartmann, M.; Lindhorst, T.K. The bacterial lectin FimH, a target for drug discovery-carbohydrate inhibitors of type 1 fimbriae-mediated bacterial adhesion. Eur. J. Org. Chem. 2011, 2011, 3583–3609.
- Bouckaert, J.; Mackenzie, J.; de Paz, J.L.; Chipwaza, B.; Choudhury, D.; Zavialov, A.; Mannerstedt, K.; Anderson, J.; Piérard, D.; Wyns, L.; et al. The affinity of the FimH fimbrial adhesin is receptor-driven and quasi-independent of Escherichia coli pathotypes. Mol. Microbiol. 2006, 61, 1556–1568.
- Han, Z.; Pinkner, J.S.; Ford, B.; Obermann, R.; Nolan, W.; Wildman, S.A.; Hobbs, D.; Ellenberger, T.; Cusumano, C.K.; Hultgren, S.J.; et al. Structure-Based drug design and optimization of mannoside bacterial FimH antagonists. J. Med. Chem. 2010, 53, 4779–4792.
- Schönemann, W.; Lindegger, M.; Rabbani, S.; Zihlmann, P.; Schwardt, O.; Ernst, B. 2-C-Branched mannosides as a novel family of FimH antagonists-synthesis and biological evaluation. Perspect. Sci. 2017, 11, 53–61.
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Proposed dual antagonist approach for the prevention and treatment of urinary tract infections caused by uropathogenic Escherichia coli. Med. Hypotheses 2019, 124, 17–20.
- Sehad, C.; Shiao, T.C.; Sallam, L.M.; Azzouz, A.; Roy, R. Effect of dendrimer generation and aglyconic linkers on the binding properties of mannosylated dendrimers prepared by a combined convergent and onion peel approach. Molecules 2018, 23, 1890.
- Touaibia, M.; Krammer, E.M.; Shiao, T.C.; Yamakawa, N.; Wang, Q.; Glinschert, A.; Papadopoulos, A.; Mousavifar, L.; Maes, E.; Oscarson, S.; et al. Sites for dynamic protein-carbohydrate interactions of O- and C-Linked mannosides on the E. coli FimH adhesin. Molecules 2017, 22, 1101.
- Kalas, V.; Hibbing, M.E.; Maddirala, A.R.; Chugani, R.; Pinkner, J.S.; Mydock-McGrane, L.K.; Conover, M.S.; Janetka, J.W.; Hultgren, S.J. Structure-Based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc. Natl. Acad. Sci. USA 2018, 115, E2819–E2828.
- Johnson, B.K.; Abramovitch, R.B. Small molecules that sabotage bacterial virulence. Trends Pharmacol. Sci. 2017, 38, 339–362.
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019, 47, 13–23.
- Bouckaert, J.; Berglund, J.; Schembri, M.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 2005, 55, 441–455.
- Mousavifar, L.; Vergoten, G.; Charron, G.; Roy, R. Comparative study of aryl O-, C-, and S-mannopyranosides as potential adhesion inhibitors toward uropathogenic E. coli FimH. Molecules 2019, 24, 3566.
- Mousavifar, L.; Touaibia, M.; Roy, R. Development of mannopyranoside therapeutics against adherent-invasive Escherichia coli infections. Acc. Chem. Res. 2018, 51, 2937–2948.
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Lüthi, C.; Scharenberg, M.; Bezençon, J.; et al. FimH antagonists for the oral treatment of urinary tract infections: From design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 2010, 53, 8627–8641.
- Han, Z.; Pinkner, J.S.; Ford, B.; Chorell, E.; Crowley, J.M.; Cusumano, C.K.; Campbell, S.; Henderson, J.P.; Hultgren, S.J.; Janetka, J.W. Lead optimization studies on FimH antagonists: Discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J. Med. Chem. 2012, 55, 3945–3959.
- Schwardt, O.; Rabbani, S.; Hartmann, M.; Abgottspon, D.; Wittwer, M.; Kleeb, S.; Zalewski, A.; Smieško, M.; Cutting, B.; Ernst, B. Design, synthesis and biological evaluation of mannosyl triazoles as FimH antagonists. Bioorg. Med. Chem. 2011, 19, 6454–6473.
- Heidecke, C.D.; Lindhorst, T.K. Iterative synthesis of spacered glycodendrons as oligomannoside mimetics and evaluation of their antiadhesive properties. Chemistry 2007, 13, 9056–9067.
- Gupta, K.; Chou, M.Y.; Howell, A.; Wobbe, C.; Grady, R.; Stapleton, A.E. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J. Urol. 2007, 177, 2357–2360.
- Hisano, M.; Bruschini, H.; Nicodemo, A.C.; Srougi, M. Cranberries and lower urinary tract infection prevention. Clinics (Sao Paulo) 2012, 67, 661–668.
- Nicolosi, D.; Tempera, G.; Genovese, C.; Furneri, P.M. Anti-Adhesion activity of A2-type proanthocyanidins (a Cranberry Major Component) on uropathogenic E. coli and P. mirabilis Strains. Antibiotics 2014, 3, 143–154.
- Scharf, B.; Sendker, J.; Dobrindt, U.; Hensel, A. Influence of cranberry extract on tamm-horsfall protein in human urine and its antiadhesive activity against uropathogenic Escherichia coli. Planta Med. 2019, 85, 126–138.
