Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Juan Carlos Romero-Benavides and Version 4 by Rodrigo Duarte-Casar.
Tragia L. is a genus of plants belonging to the Euphorbiaceae family with worldwide intertropical distribution, composed of more than 150 species.
- Tragia L.
- Genus
- Distribution
- Biological Activity
- Composition
1. Genus
The genus Tragia is one of the 317 genera in the Euphorbiaceae family. There are 161 accepted names belonging to 154 species in the Tragia genus, with “pantropical and warm temperate distribution” [1][2][9,10]. The etymology for the name of this genus comes from the Greek tragos, meaning goat. This name may stem either from the name of the German botanist Hieronymus Bock—Bock means “ram” or “he-goat” in German, or from the hairy appearance of the plant that would resemble a male goat [3][11].
Tragia species exhibit very ample morphological characters: they are perennial plants with herb, shrub, subshrub and twining vine growth habits, with lanceolate leaves presenting either entire or serrated margins. Plants belonging to this genus sting when touched due to the presence of leaf hairs with a needle-shaped crystal of calcium oxalate (raphide) in the terminal cells that is expelled on contact and punctures the skin, allowing irritants to enter and cause transient stinging [4][5][12,13], presumably a defense mechanism against herbivores [6][14]. Several common names for Tragias, such as noseburn (Tragia spp.), Indian stinging nettle (T. involucrata), fireman (T. volubilis) or stinging nettle creeper (T. durbanensis), are due to this stinging property.
Species belonging to Euphorbiaceae in general and to Tragia in particular are still not fully settled [7][8], as new species are being discovered [8][15] and species are being reassigned to other genera [1][9][9,16], so the number of species in the genus is still subject to change.
2. Distribution and Localization
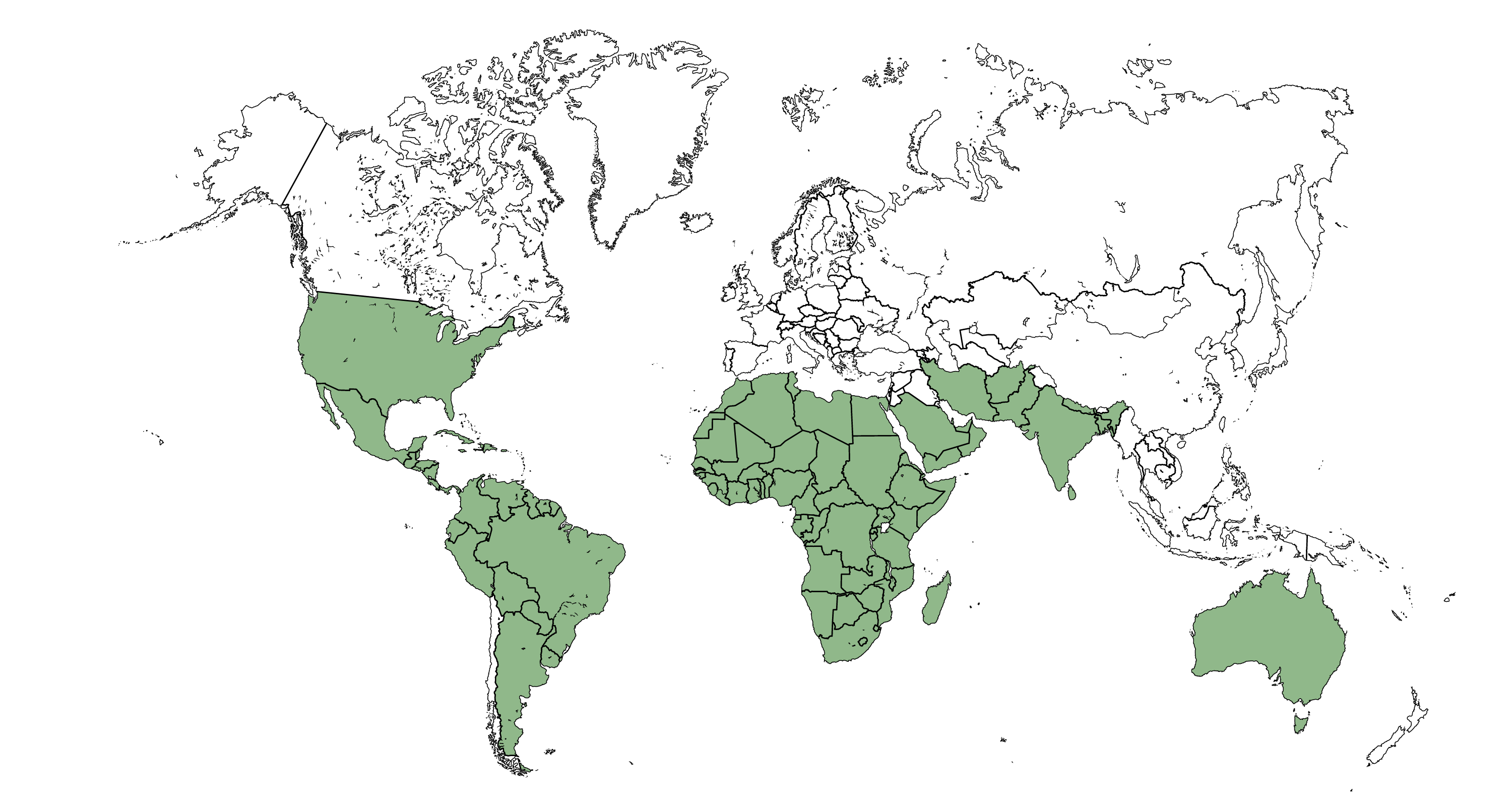
Species belonging to the Tragia genus are present in subtropical America, Eastern and Southern Africa, the Indian subcontinent and Northeastern Australia. Of the 154 species listed in the genus [10][17], 94 are found in Africa, 48 in America, 10 in Asia and 3 in Oceania, with some species such as T. arabica and T. plukenetii present both in Africa and Asia.
3. Ethnopharmacological Usage
4. Biological Activity
4.1. In Vitro Activity
4.2. In Vivo Activity
5. Phytochemical Composition
Table 1. Compounds isolated/identified in Tragia extracts and oils and their biological effect.
| No. | Compound | Identified | Isolated | Methodology Used | Species | Collection area | Plant Organ Used | Use | Effect | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetal | ||||||||||||||||
| 1 | 1,1-diethoxy-2- methylpropane | X | Ethanol extract GC, MS |
T. plukenetii | NS | Whole plant | NS | NS | [21][111] | |||||||
| Aldehydes | ||||||||||||||||
| 2 | 16-heptadecenal | X | Ethanol extract GC, MS |
T. plukenetii | NS | Whole plant | NS | NS | [21][111] | |||||||
| 3 | Hexanal | X | Hydrodistillation GC/GC-MS |
T. benthamii | Ibadan, Nigeria | Leaves | NS | NS | [22][112] | |||||||
| Alkaloid | ||||||||||||||||
| 4 | (E)-4-(1-hydroxypropyl)-7,8-dimethyl-9-(prop-1-en-1-yl)-[1,3] dioxolo [4,5-g]quinolin-6(5 | H | )-one | X | X | Acidified ethanol extract GC, MS, LC |
T. plukenetii | NS | Whole plant | NS | NS | [21] | [111] | |||
| Esters | ||||||||||||||||
| 5 | 4-oxo-4H-pyran-2,6-dicarboxylic acid bis-[6-methyl-heptyl] ester | X | X | Ethanol extract IR 1H, 13C NMR, MS |
T. involucrata | Salem, India | Roots | Antidiabetic | Blood glucose reduction | [23][86] | ||||||
| 6 | Ethyl linoleate | X | X | Ethanol extract GC, MS |
T. plukenetii | NS | Whole plant | NS | NS | [21][111] | ||||||
| 7 | Ethyl palmitate | X | X | Ethanol extract GC, MS |
T. plukenetii | NS | Whole plant | NS | NS | [21][111] | ||||||
| Ether | ||||||||||||||||
| 8 | Vinyl hexyl ether | X | X | Aqueous extract GC, MS |
T. involucrata | Tamil Nadu, India | Leaf | Antibacterial | Escherichia coli | Proteus vulgaris | Staphylococcus aureus | MBC 12.25 μg/mL | [24][25] | [98,113] | ||
| Flavonoids | ||||||||||||||||
| 9 | 3-(2,4-dimethoxyphenyl)-6,7-dimethoxy-2,3-dihydrochromen-4-one | X | X | Ethyl acetate extract FTIR, MS, 1H NMR |
T. involucrata | Odisha, India | Root | Antibacterial Fungicidal |
MIC 1.25-12.5 μg/mL | [26][53] | ||||||
| 10 | Iridin | X | X | Ethyl acetate extract FTIR, MS, 1H NMR |
T. involucrata | Odisha, India | Root | Toxic | [26][53] | |||||||
| 11 | Quercetin | X | X | Ethyl acetate extract FTIR, MS, 1H NMR |
T. involucrata | Odisha, India | Root | Antioxidant | [26][53] | |||||||
| 12 | Rutin | X | X | Ethyl acetate extract FTIR, MS, | 1 | H NMR | T. involucrata | Odisha, India | Root | Antioxidant | [26] | [53] | ||||
| Heterocycle | ||||||||||||||||
| 13 | 2,5-dithia-3,6-diazabicyclo[2.2.1]heptane | X | X | 95% aqueous ethanol extraction | 1 | H, | 13 | C NMR | T. benthamii | Ibadan, Nigeria | Whole plant | NS | [27] | [114] | ||
| Hydrocarbons | ||||||||||||||||
| 14 | 2,6-dimethylheptane | X | X | Aqueous extract GC, MS |
T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Proteus vulgaris |
MBC 10 μg/mL | [24][98] | ||||||
| 15 | 2,4-dimethylhexane | X | X | Aqueous extract GC, MS |
T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Staphylococcus aureus |
MBC 12.25 μg/mL | [24][98] | ||||||
| 16 | 2-methylnonane | X | X | Aqueous extract GC, MS |
T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Escherichia coli Proteus vulgaris Staphylococcus aureus |
MIC 5.0 μg/mL | [24][98] | ||||||
| 17 | Shellsol (2-methyldecane) |
X | X | Aqueous extract GC, MS |
T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Proteus vulgaris Staphylococcus aureus |
MBC 25.0 μg/mL | [24][98] | ||||||
| 18 | 3,5-di-tert-butyl-4-hydroxyanisole | X | X | 95% aqueous ethanol extraction 1H, 13C NMR |
T. benthamii | Ibadan, Nigeria | Whole plant | Antioxidant | [27][114] | |||||||
| 19 | 5-hydroxy-1-methylpiperdin-2-one | X | X | Methanol extract IR, | 1 | H, | 13 | C RMN, LC | T. involucrata | Kerala, India | Leaf | Antihistamine | Muscle relaxant, bronchodilating and anti-allergic effects | [28] | [115] | |
| Polyols | ||||||||||||||||
| 20 | Erythritol | X | X | 95% aqueous ethanol extraction 1H, 13C NMR |
T. benthamii | Ibadan, Nigeria | Whole plant | NS | NS | [27][114] | ||||||
| 21 | Glycerol | X | X | 95% aqueous ethanol extraction | 1 | H, | 13 | C NMR | T. benthamii | Ibadan, Nigeria | Whole plant | NS | NS | [27] | [114] | |
| Terpenoids | ||||||||||||||||
| 22 | 10,13-dimethoxy-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[α]phenanthrene. | X | X | Ethyl acetate extract FTIR, MS, 1H NMR |
T. involucrata | Odisha, India | Root | NS | NS | [26][53] | ||||||
| 23 | Stigmasterol | X | Aqueous extract GC, MS |
T. involucrata | Leaf | NS | NS | [24][98] | ||||||||
| 24 | Caryophyllene | X | Hydrodistillation GC/GC-MS |
T. benthamii | Ibadan, Nigeria | Leaves | Anti inflammatory | [22][112] | ||||||||
| 25 | Citronellal | X | X | Ethanol extract IR, 1H RMN, LC |
T. ramosa | Maharashtra, India | Leaves | Antibacterial | [29][71] | |||||||
| 26 | Clerodane | X | X | Ethanol extract IR, 1H RMN, LC |
T. ramosa | Maharashtra, India | Leaves | NS | NS | [29][71] | ||||||
| 27 | Geranylacetone | X | Hydrodistillation GC/GC-MS |
T. benthamii | Ibadan, Nigeria | Leaves | NS | NS | [22][112] | |||||||
| 28 | Neophytadiene (2-(4,8,12-Trimethyltridecyl) buta-1,3-diene) | X | X | Ethanol extract GC, MS |
T. plukenetii | NS | Whole plant | NS | NS | [21][111] | ||||||
| 29 | Phytol | X | X | 95% aqueous ethanol extraction 1H, 13C NMR |
T. benthamii | Ibadan, Nigeria | Whole plant | NS | NS | [27][114] | ||||||
| 30 | Squalene (all trans) | X | X | Ethanol extract GC, MS |
T. plukenetii | NS | Whole plant | NS | NS | [21][111] | ||||||
| 31 | α-terpinene | X | X | Ethanol extract IR, | 1 | H RMN, LC | T. ramosa | Maharashtra, India | Leaves | Antiinflammatory, Antimicrobial |
NS | [29] | [71] | |||
