Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jerzy Palka and Version 2 by Yvaine Wei.
The estrogen receptor (ER) status and the availability of agonists or antagonists of these receptors determine the processes of growth, differentiation, and proliferation of breast cancer cells. Estrogens and anti-estrogenic compounds have been shown to influence breast cancer cell survival/apoptosis via action through the mitochondrial enzyme proline dehydrogenase/proline oxidase (PRODH/POX).
- estrogens
- estrogen receptor
- breast cancer
1. Introduction
Breast cancer was the most common malignant neoplasm in women and accounted for 11.7% of all cancers globally. WHO cites obesity as one of the main reasons for the high incidence of the disease. The recent increase in the mortality of breast cancers was due to the COVID-19 pandemic that affected both therapy and prevention of the disease [1][2][1,2]. Although several therapeutic approaches for breast cancer treatment have been established, the role of estrogen receptor (ER) status in the complex regulatory mechanisms driving apoptosis/survival of cancer cells is not fully understood.
The presence of the ER (ER+) in breast cancers increases positive response to anticancer treatment. Moreover, a better prognosis concerns progesterone receptors (PR+) and human epidermal growth factor (HER2+) positive cancers. The absence of ER is a significant risk factor for relapse and shorter life expectancy. Some authors emphasize that at least a two-receptor ER+PR+HER- expansion profile has a better prognosis than a single-receptor profile such as ER+PR-HER- or ER-PR+HER- [3]. This is probably due to the hormonal reorganization of cellular metabolism driving pro-survival or pro-apoptotic pathways. However, the mechanisms driving apoptosis/survival are not fully understood.
2. Estrogen Receptors Structure, Location and Function
Two distinct estrogen receptor (ER) types, ERα and ERβ, are known to be encoded by two different genes located on two different chromosomes. ERα and ERβ are encoded by ESR1 (chromosome 6, region q24-q27) and ESR2 gene (chromosome 14, region q23.2). The molecular weight of ERα is 67 kDa, the ERβ isoform has 57 kDa [4]. Both types are composed of 6 functional domains named A–F [5]. Domains A and B are located at the amino terminal of the protein. The domain AF1 is able to activate gene transcription in the absence of bound ligand (e.g., the estrogen); however, the activation is weak. Domain C is responsible for receptor dimerization and binding of the ligand-receptor complex to a specific sequence on DNA. The D domain is also called the hinge. It has DNA-binding properties, and its sequence is more variable than that of the C domain. Next is the E domain, which contains a hydrophobic pocket structure called the ligand-binding domain (LBD). The E domain also enables dimerization of nuclear receptors. Some receptors also have an F domain, whose role is not fully elucidated (Figure 1) [5].
Figure 1. The structure of the estrogen receptor. ERα—Estrogen Receptor α; ERβ—Estrogen Receptor β; AF1—activator of transcription 1; C-DBD—DNA Binding Domain, domain C; D-H—Domain D-hinge; E-LBD—Ligand Binding Domain, domain E; AF-2—activator of transcription 2; NH2—amino-terminus, NH2—terminus, N—terminal end or amine-terminus; COOH—carboxylic terminus.

Figure 2. ER-dependent gene transcription. E2—estradiol; ER—Estrogen Receptor, HSP—Heat Shock Proteins; FoxA1—Forkhead box protein A1; ERE—Estrogen Response Element; mRNA—messenger RNA.
The distribution of ERα and ERβ receptors in tissues and organs varies. In most tissues and organs, both types of estrogen receptors are present, while in some, only one type predominates. In the ovaries, uterus, mammary gland, kidney, adrenal gland, testes, epididymis, pituitary gland, and hypothalamus, ERα expression is higher [7][8][9][7,8,9] than in the urinary bladder, prostate gland, heart, and liver [10]. The highest level of ERβ expression was found in the ovary and prostate gland [11]. An important function of estrogen receptors is transcriptional and post-transcriptional regulation of cellular metabolism [12]. It has been suggested that ERα is involved in the regulation of cell proliferation, while ERβ evokes anti-proliferative and pro-apoptotic activity [13][14][13,14]. However, ERs comprise also several membranes bound receptors as G protein-coupled estrogen receptor (GPER) and Gq-coupled membrane estrogen receptor (GqmER). Recent studies revealed a functional link between all types of ERs. Interestingly, several oncogenic miRNAs have been shown to modulate the expression of ERs affecting malignant behaviour of cancer cells [15]. Moreover, a ligand-independent signaling has been reported for ERα through kind of cross-talk with epidermal growth factor or insulin-like growth factor-I [16][17][16,17]. Whether they are involved in PRODH/POX-dependent regulation of apoptosis/survival requires to be explored.
3. Apoptosis
Apoptosis is the process of programmed cell death, important in the development and homeostasis of multicellular organisms [18]. This process enables the elimination of damaged, old or unnecessary cells. Initiation of the apoptosis pathway is one of the possible cell responses to intracellular or extracellular action of the chemical, physical or biological factors. The external factors that cause cell damage include UV radiation, ionizing radiation, thermal shock, low availability of oxygen and nutrients, drugs, or viral and bacterial infections [19]. The internal factors are activated by oncogenes, cell cycle defects, deficiency of growth factors, energy, hormonal deregulation, etc. [20][21][20,21]. Factors inducing apoptosis contribute to the development of neurodegenerative and autoimmune diseases, growth defects, and cancer. The disturbed balance between survival and apoptosis is a common feature of cancer cells [22]. It is also the cause of resistance to chemotherapy, radiotherapy, hormonal and immune therapy [23].
Apoptosis is a precisely regulated process by several classes of proteins. The most important are caspases (a family of intracellular cysteine proteases). They are divided into initiator, implementing, and inflammation caspases. Another important protein in the apoptosis process is the family of BCL-2 proteins (Bax; Bak, Bid, Bim), which have proapoptotic, antiapoptotic, and regulatory activities [24].
Several pathways lead to the induction of apoptosis. The extrinsic pathway is initiated by binding a ligand to the death surface receptors [25]. The intrinsic pathway of apoptosis can be activated by proapoptotic factors released from mitochondria. Apoptogenic molecules that are produced during intracellular stress leads to the increase in permeation of mitochondria. Both pathways stimulate apoptosis through proteolytic cleavage of pro-caspases into active enzymes [26]. The initiator caspases include caspase-8, -9, -10, whereas caspases-3, -6, and -7 are called effector caspases [27]. They can disrupt entire cells within a few minutes.
3.1. The Extrinsic Apoptosis Pathway
The extrinsic process of apoptosis is induced in the cell through the signals from other cells activating the death receptor, which initiates a cascade of intracellular effector proteins [28][29][28,29]. Tumor necrosis factor (TNF) is the best-characterized protein that initiates programmed cell death [30]. The same superfamily includes ligand of TNF family receptors (THANK), lymphotoxin (LT), Fas Ligand (FasL), TNF-related apoptosis-inducing ligand (TRAIL), or the Vascular Endothelial Growth Inhibitor (VEGI) [31]. Some of them contain an intracellular death domain (DD). During protein binding to the receptors of the TNF family, the TRADD (Tumor necrosis factor receptor type 1-associated DEATH domain protein) or FADD (Fas-associated protein with death domain) adapter proteins interact with the DD region. Subsequently, the DISC complex (Death-inducing signaling complex) is formed [32][33][34][32,33,34]. This complex combines procaspases -8 and -10 and has autoproteolytic activation properties [35]. Cleaved caspases -8 and -10 activate the implementing caspases and initiate changes in the cell structure leading to cell death [32]. In addition, active caspases -8 and -10 activate BID (a pro-apoptotic BCL family protein), which leads to increased release of cytochrome C from mitochondria by its truncated form tBID (Figure 3).
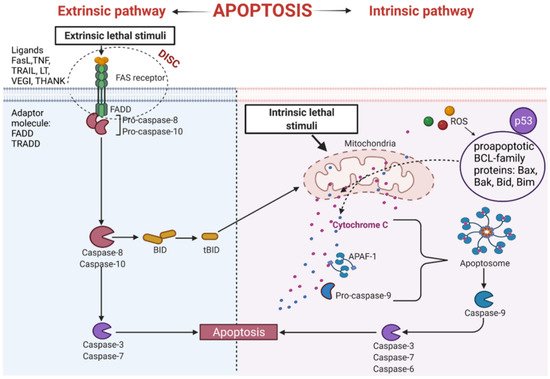

Figure 3. Intrinsic and extrinsic apoptotic pathway. THANK—TNF family receptor; LT—lymphotoxin; FasL—Fas Ligand; TRAIL—TNF-related apoptosis-inducing ligand; VEGI—Vascular Endothelial Growth Inhibitor; TNF—tumor necrosis factor; DISC—Death-inducing signaling complex; FADD—Fas-associated protein with death domain); TRADD—tumor necrosis factor receptor type 1-associated DEATH domain protein; p53—tumor protein p53; APAF-1—apoptotic protease activating factor-1; ROS—reactive oxygen species; Bax, Bak, Bid, Bim—proapoptotic BCL-family proteins; tBID—truncated BID.
An important apoptosis inducer is a p53 protein. This protein participates in the external and internal pathways of apoptosis. p53 interacts with BCL (B-cell lymphoma) proteins family contributing to the upregulation of mitochondrial channels and the cytochrome C efflux into the cytoplasm, activating the internal pathway of programmed cell death [36][37][36,37]. It has been established that p53 also induces genes coding for death receptors and death ligands [37].
3.2. The Intrinsic Apoptosis Pathway
This pathway is also called a mitochondrial pathway. It depends on energetic and metabolic processes in the cells and is induced by stress factors. These factors are oxidative stress, DNA damage, changes in cytoplasmic calcium ions concentration, and others. Furthermore, the production of reactive oxygen species (ROS) activates pro-apoptotic BCL- family proteins [38]. As a result of these reactions, the mitochondrial membrane is leaking [39], leading to the release of cytochrome C from mitochondria [38]. Released cytochrome C binds with procaspase9 and apoptotic protease activating factor-1 (APAF-1), forming apoptosome complex. The complex activates the cascade of structural changes in the cell that contribute to cell death through active forms of executive caspases such as caspase-3, caspase-6, and caspase-7 (Figure 3) [40][41][40,41].
