Antibiotic resistance is a serious threat to public health and new antimicrobial compounds are urgently needed. One of the most promising classes for the development of new antimicrobials are plant-derived alkaloids.
- antibiotic resistance
- antimicrobials
- alkaloids
- methicillin-resistant Staphylococcus aureus
- vancomycin-resistant enterococci
- natural products
- plant-derived alkaloids
- structure–activity relationship
Antibiotic resistance is now considered a worldwide problem that puts public health at risk. The onset of bacterial strains resistant to conventional antibiotics and the scarcity of new drugs have prompted scientific research to re-evaluate natural products as molecules with high biological and chemical potential. A class of natural compounds of significant importance is represented by alkaloids derived from higher plants.
- Introduction
1. Introduction
The discovery and the advent of penicillin in clinical practice have led to the subsequent discovery of numerous new antibiotics to be used as an invaluable weapon against bacterial infections. However, the beginning of the antibiotic era coincided with the onset and characterization of antibiotic-resistant strains. This triggered the entrance into our current post-antibiotic era in which fewer and fewer antibiotics are discovered at the expense of a high occurrence of multidrug resistant (MDR) infections [1]. Currently in Europe, the number of MDR infections accounts to 700 thousand and provokes 33,000 deaths every year, resulting in an estimated cost of above €1.5 billion for their treatment [2]. These infections are a real threat to global public health and numerous efforts are underway to contain the spread of MDR strains, particularly in hospital settings and cities with high population [3]. A very recent example of a pandemic threat is represented by infections caused by Gram-positive methicillin resistant Staphylococcus aureus (MRSA) strains and vancomycin resistant enterococci (VRE). The first case of MRSA was identified in the early 60s and currently this infection appears at high incidence in Europe, Asia and America [4]. In the latter, MRSA infections provoke more deaths annually than AIDS, emphysema and homicides [5]. MRSA strains can be classified into hospital-acquired MRSA (HA-MRSA) and community-acquired MRSA (CA-MRSA), according to their original sources, but more recently, several MRSA strains resulted to be not strictly related to health care-associated infections, e.g., MRSA associated to livestock (LA-MRSA) [6][7][8]. The other challenge of public health is given by VRE infections. These are commonly caused by Enterococcus faecium and Enterococcus faecalis and provoke surgical-site, urinary tract and bloodstream infections [9]. Although with a lower incidence than MRSA, VRE cause about 66,000 infectious cases in the U.S. annually. Another example of dangerous infections is represented by carbapenem-resistant Enterobacteriaceae (CRE), a group of bacteria such as Klebsiella pneumoniae and Escherichia coli, which produce enzymes (e.g., New Delhi metallo-beta-lactamase, NMD-1) able to make them resistant to virtually all beta-lactams. As of February 2019, the US Food and Drug Administration (FDA) approved some antibiotic drugs i.e., ceftazidime-avibactam, meropenem-vaborbactam, plazomicin and eravacycline for treatment of some CRE-related infections [10]. In light of these difficult resistant infections and the scarcity of new approved antibiotics, it is now evident that research must open the horizons to new therapeutic strategies, new combinatorial therapies of drugs and to the discovery of new antimicrobials [11][12][13].
The Need for New Antimicrobials
Antibiotics have drastically changed people’s lives: in America, in 1920, life expectancy was 56 years while in 2020 it was around 80; indeed, in developing countries, antibiotics have reduced the morbidity and mortality caused by food-borne and poverty-related diseases [8].
However, large pharmaceutical companies averaged a drastic decline in the production of new antibiotics [14]. This is because the economic crisis at the beginning of the century has led to substantial cuts in academic research and health care spending; in addition, pharmaceutical industries have been more oriented towards investment of drugs capable of curing chronic diseases, which translate into greater economic revenues. It is worthwhile noting that in America a chemotherapy treatment can cost tens of thousands of dollars, compared to about 3,000 of an antibiotic therapy [15]. In addition, the easy availability of antibiotics and the relatively low costs make them of little value to consumers. It follows that a new antibiotic drug should not cost much more, to be purchased. Finally, regulation for clinical trials has become much more complex: studies with antibiotics and placebo are now considered unethical, so trials can only be conducted to demonstrate better drug activity than an existing antibiotic. This results in longer and more expensive clinical trials [15]. Moreover, once on the market, the antibiotic may become useless by the appearance of resistance. As a consequence, new strategies and new sources of antimicrobial molecules are highly demanded.
Nature is undoubtedly the richest source of molecules with the most varied biological features. Due to its biodiversity not only between animal and plant kingdoms, but also among the various species, nature represents the largest library of compounds that has ever existed [16][17][18].
Many of these molecules exhibit antimicrobial activity and have a chemical structure often very different from each other. Examples are antimicrobial peptides produced by insects, amphibians, mammals and plants [12][19][20][21]. These can have a cyclic or a linear structure, and consist of no more than 50 amino acids and have various biological properties, from an antimicrobial to an immunomodulatory function [22][23][24][25]. Another promising class of natural compounds from the plant kingdom is given by secondary metabolites. Many of these (e.g., tannins, terpenes, carotenoids, polyphenols and alkaloids) have already been characterized for their biological properties and relevance as potential new antimicrobials [26][27][28][29][30]. Furthermore, these molecules can serve as a chemical scaffold for the synthesis of libraries in order to identify markers for a specific detection or for the design of lead compounds with a desired biological activity [31][32][33].
- Alkaloids
2. Alkaloids
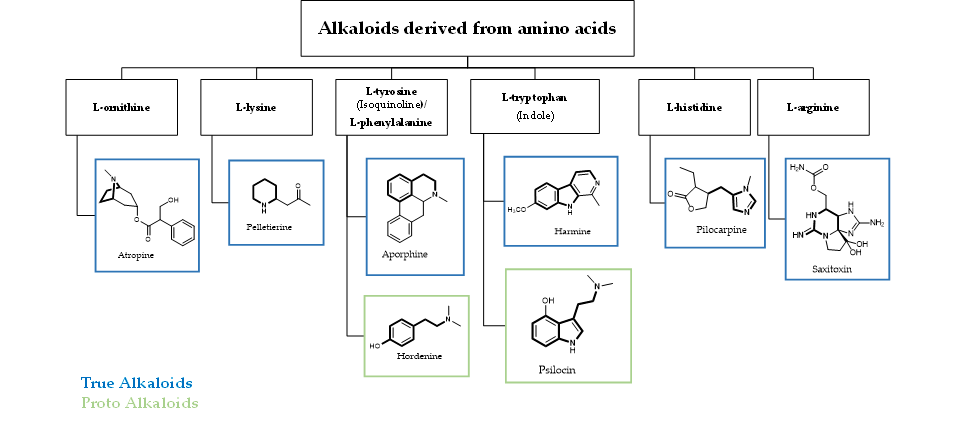
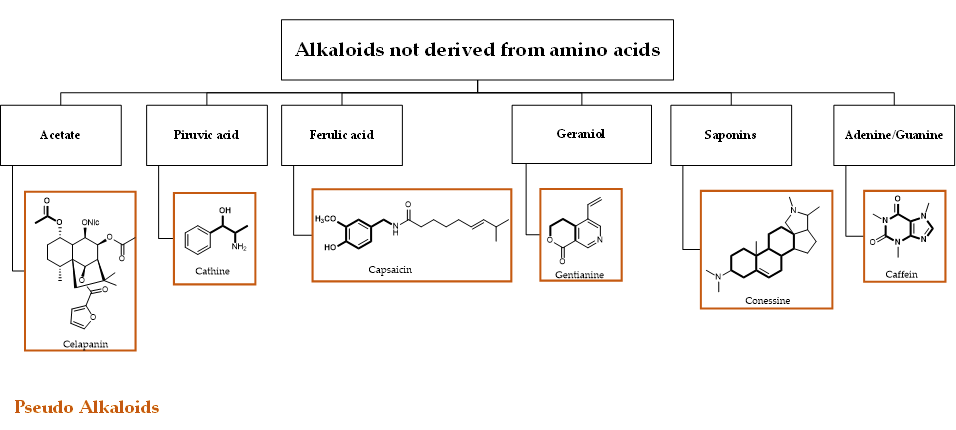
Alkaloids represent a wide and structurally diverse group of secondary metabolites that can be found in 300 plant families, as well as in bacteria, fungi and animals [34]. To date, more than 18,000 different alkaloids have been discovered [35][36]. The name ‘alkaloid’ (alkali-like) is due to their basic nature, which allows them to be found as salts of organic acid or free bases. An individual alkaloid name consists of the permanent suffix ‘-ine’, linked to their amino origin, and by a more changeable prefix. This can be named after pharmacological activities (e.g., emetine), their discoverer (e.g., pelletrine) and the source’s geographic location from which they were isolated (e.g., atropine) [37][38]. Alkaloids are characterized by great structural diversity; the sole unifying feature is the presence of a basic nitrogen atom that can occur in the form of a primary amine (RNH2), a secondary amine (R2NH) or a tertiary amine (R3N). They can occur as monomers or they can form oligomers (homo or hetero-oligomers). Although there is no standard taxonomic classification, alkaloids can generally be classified according to their chemical structure, biochemical pathway or natural origin [39]. From a biosynthetically point of view, alkaloids can be divided into three major categories: true-, proto- and pseudo-alkaloids (Figures 1 and 2).
Figure 1. Schematic representation of true- and proto-alkaloids. The amino acidic skeleton derived from the natural precursor is in bold.
Figure 2. Schematic representation of pseudo-alkaloids. The carbon skeleton derived from the natural precursor is in bold.
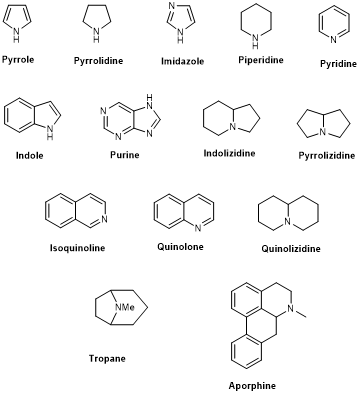
True- and proto-alkaloids have an amino acid as a precursor, but they differ for the presence or not of the N-atom in the heterocycle, respectively. Pseudoalkaloids feature a basic carbon skeleton not deriving from an amino acid [40]. Alkaloids are often classified on the basis of their chemical structure in heterocyclic or typical alkaloids (true alkaloids), containing nitrogen in the heterocycle, and non-heterocyclic or atypical (proto-alkaloids), containing nitrogen in a side chain. Since their structural complexity and according to their backbone, heterocyclic alkaloids can be split into 14 subgroups including indoles, isoquinolines, pyrrolizidines, pyrrolidines, quinolizidines, tropanes, purines, piperidines and imidazoles (Scheme 1)[36].
Scheme 1. The 14 subgroups of alkaloids.
Alkaloids have been extensively investigated for their biological activity (e.g., anticancer, antibacterial, antiviral and central nervous depressant activity) in both traditional and modern medicine [41]. Notably, their exceptional biological activity is provided by the ability to form hydrogen bonds with enzymes, receptors and proteins due to the presence of a proton accepting nitrogen atom and one or more protons donating amine hydrogen atoms. In recent years, the alkaloids’ antibacterial activity played a significant role in the treatment of many infectious diseases reporting MDR phenomena. This led researchers to direct their attention onto these promising plant secondary metabolites [42]. Thus, the development of different extraction methods to obtain pure alkaloids results to be very important, even if they are often produced in very small amounts by their natural source and their enantioselective separation is quite difficult, mostly due to the presence of a large number of chiral centers. In order to overcome these issues, a wide range of synthetic efforts has been recorded with the aim to achieve enantiomerically pure alkaloids [43]. One of the most direct, efficient, and variable synthetic methods for the construction of privileged pharmacophores (i.e., tetrahydro-isoquinolines, tetrahydro-β-carbolines and polyheterocyclic frameworks) and for the creation of natural compounds libraries in medicinal chemistry proved to be the Pictet-Spengler reaction [44][45]. This reaction, in combination with chiral catalysts, has been reported in the total synthesis of complex alkaloids [46]. Another synthetic approach widely employed for the construction of sophisticated macromolecules architecture, such as alkaloids, is the olefin metathesis reaction, which is one of the most powerful tools for the formation of challenge polycyclic frameworks and bridged nitrogen heterocycles [47][48][49]. Most of the alkaloids reported below are known and their multiple chiral centers were assigned according to the literature.
- Conclusions
3. Conclusions
The drastic drop in the number of new antibiotics on the market has led scientific research to reassess nature as an invaluable source of biologically active compounds. Among these, alkaloids of plant origin represent an interesting example of compounds for their biological and chemical properties. In this review we highlighted the potential of these alkaloids as antimicrobials specifically against strains resistant to conventional antibiotics or as adjuvants to be used in combination. The various data reported here have clearly shown that alkaloids can also be used as chemical scaffolds for further structural modifications. Taken all together, the data collected in this manuscript reinforce the idea that alkaloids can be considered as new alternative antimicrobials.
References
- Andrew F. Read; Robert J. Woods; Antibiotic resistance management. Evolution, Medicine, and Public Health 2014, 2014, 147-147, 10.1093/emph/eou024.
- Alessandro Cassini; Liselotte Diaz Högberg; Diamantis Plachouras; Annalisa Quattrocchi; Ana Hoxha; Gunnar Skov Simonsen; Mélanie Colomb-Cotinat; Mirjam E Kretzschmar; Brecht Devleesschauwer; Michele Cecchini; et al.Iss Ait OuakrimTiago Cravo OliveiraMarc J StruelensCarl SuetensDominique L MonnetReinhild StraussKarl MertensThomas StruyfBoudewijn CatryKatrien LatourIvan N IvanovElina G DobrevaArjana Tambic AndraševicSilvija SoprekAna BudimirNiki PaphitouHelena ŽemlickováStefan Schytte OlsenUte Wolff SönksenPille MärtinMarina IvanovaOuti LyytikäinenJari JalavaBruno CoignardTim EckmannsMuna Abu SinSebastian HallerGeorge L DaikosAchilleas GikasSotirios TsiodrasFlora KontopidouÁkos TóthÁgnes HajduÓlafur GuólaugssonKarl G KristinssonStephen MurchanKaren BurnsPatrizio PezzottiCarlo GagliottiUga DumpisAgne LiuimieneMonique PerrinMichael A BorgSabine C De GreeffJos Cm MonenMayke Bg KoekPetter ElstrømDorota ZabickaAleksander DeptulaWaleria HryniewiczManuela CaniçaPaulo Jorge NogueiraPaulo André FernandesVera ManageiroGabriel A PopescuRoxana I SerbanEva SchréterováSlavka LitvováMária ŠtefkovicováJana KolmanIrena KlavsAleš KorošecBelén AracilAngel AsensioMaría Pérez-VázquezHanna BillströmSofie LarssonJacqui S ReillyAlan JohnsonSusan HopkinsBurden of AMR Collaborative Group Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis.. The Lancet Infectious Diseases 2018, 19, 56-66, 10.1016/S1473-3099(18)30605-4.
- Zhabiz Golkar; Omar Bagasra; Donald Gene Pace; Bacteriophage therapy: a potential solution for the antibiotic resistance crisis.. The Journal of Infection in Developing Countries 2014, 8, 129-136, 10.3855/jidc.3573.
- Yunlei Guo; Guanghui Song; Meiling Sun; Juan Wang; Yi Wang; Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Frontiers in Cellular and Infection Microbiology 2020, 10, 107, 10.3389/fcimb.2020.00107.
- Michael Gross; Antibiotics in crisis. Current Biology 2013, 23, R1063-R1065, 10.1016/j.cub.2013.11.057.
- Michael Otto; Community-associated MRSA: What makes them special?. International Journal of Medical Microbiology 2013, 303, 324-330, 10.1016/j.ijmm.2013.02.007.
- Jodi A. Lindsay; Hospital-associated MRSA and antibiotic resistance—What have we learned from genomics?. International Journal of Medical Microbiology 2013, 303, 318-323, 10.1016/j.ijmm.2013.02.005.
- Gian Maria Rossolini; Fabio Arena; Patrizia Pecile; Simona Pollini; Update on the antibiotic resistance crisis. Current Opinion in Pharmacology 2014, 18, 56-60, 10.1016/j.coph.2014.09.006.
- Levitus, M.; Rewane, A.; Perera, T.B.. Vancomycin-Resistant Enterococci (VRE); StatPearls: FL, USA, 2020; pp. 1.
- Shio-Shin Jean; International Society of Antimicrobial Chemotherapy (ISAC); Ian M. Gould; Wen-Sen Lee; Po-Ren Hsueh; New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship. Drugs 2019, 79, 705-714, 10.1007/s40265-019-01112-1.
- Raphaël E. Duval; Marion Grare; Béatrice Demoré; Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria.. Molecules 2019, 24, 3152, 10.3390/molecules24173152.
- Bruno Casciaro; Maria Rosa Loffredo; Vincenzo Luca; Walter Verrusio; Mauro Cacciafesta; Maria Luisa Mangoni; Esculentin-1a Derived Antipseudomonal Peptides: Limited Induction of Resistance and Synergy with Aztreonam. Protein & Peptide Letters 2019, 25, 1155-1162, 10.2174/0929866525666181101104649.
- Tony Kirby; New antimicrobials—lots of talk, where is the action?. The Lancet Infectious Diseases 2016, 16, 411-412, 10.1016/s1473-3099(16)00140-7.
- Zappia, G.; Ingallina, C.; Ghirga, F.; Botta, B.. Oxazolidin-2-Ones: Antibacterial Activity and Chemistry; Springer Berlin Heidelberg: Germany, 2014; pp. 247–266.
- C. Lee Ventola; The Antibiotic Resistance Crisis. P & T : a peer-reviewed journal for formulary management 2015, 40, 277-283.
- Filipa Barbosa; Eugénia Pinto; Anake Kijjoa; Madalena Pinto; Emília Sousa; Targeting antimicrobial drug resistance with marine natural products. International Journal of Antimicrobial Agents 2020, 0, 106005, 10.1016/j.ijantimicag.2020.106005.
- Alan L. Harvey; RuAngelie Edrada-Ebel; Ronald J. Quinn; The re-emergence of natural products for drug discovery in the genomics era. Nature Reviews Drug Discovery 2015, 14, 111-129, 10.1038/nrd4510.
- Francesca Ghirga; Alessandra Bonamore; Lorenzo Calisti; Ilaria D’Acquarica; Mattia Mori; Bruno Botta; Alberto Boffi; Alberto Macone; Green Routes for the Production of Enantiopure Benzylisoquinoline Alkaloids. International Journal of Molecular Sciences 2017, 18, 2464, 10.3390/ijms18112464.
- Bruno Casciaro; Ivana D’Angelo; Xiaoping Zhang; Maria Rosa Loffredo; Gemma Conte; Floriana Cappiello; Fabiana Quaglia; Yuan-Pu Peter Di; Francesca Ungaro; Maria Luisa Mangoni; et al. Poly(lactide-co-glycolide) Nanoparticles for Prolonged Therapeutic Efficacy of Esculentin-1a-Derived Antimicrobial Peptides against Pseudomonas aeruginosa Lung Infection: in Vitro and in Vivo Studies. Biomacromolecules 2019, 20, 1876-1888, 10.1021/acs.biomac.8b01829.
- Bruno Casciaro; Floriana Cappiello; Maria Rosa Loffredo; Francesca Ghirga; Maria Luisa Mangoni; The Potential of Frog Skin Peptides for Anti-Infective Therapies: The Case of Esculentin-1a(1-21)NH2. Current Medicinal Chemistry 2020, 27, 1405-1419, 10.2174/0929867326666190722095408.
- Brian P. Lazzaro; Michael Zasloff; Jens Rolff; Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480, 10.1126/science.aau5480.
- Annarita Falanga; Ersilia Nigro; M G De Biasi; Aurora Daniele; G. Morelli; Massimiliano Galdiero; Olga Scudiero; Cyclic Peptides as Novel Therapeutic Microbicides: Engineering of Human Defensin Mimetics. Molecules 2017, 22, 1217, 10.3390/molecules22071217.
- Bruno Casciaro; Qiao Lin; Sergii Afonin; Maria Rosa Loffredo; Valeria De Turris; Volker Middel; Anne S. Ulrich; Yuanpu Peter Di; Maria Luisa Mangoni; Inhibition of Pseudomonas aeruginosa biofilm formation and expression of virulence genes by selective epimerization in the peptide Esculentin‐1a(1‐21) NH 2. FEBS Journal 2019, 286, 3874-3891, 10.1111/febs.14940.
- Vishal Musale; Bruno Casciaro; Maria Luisa Mangoni; Yasser H. A. Abdel Wahab; Peter R Flatt; J. M. Conlon; Assessment of the potential of temporin peptides from the frog Rana temporaria (Ranidae) as anti-diabetic agents. Journal of Peptide Science 2018, 24, e3065, 10.1002/psc.3065.
- Muthuirulan Pushpanathan; Paramasamy Gunasekaran; Jeyaprakash Rajendhran; Antimicrobial Peptides: Versatile Biological Properties. International Journal of Peptides 2013, 2013, 1-15, 10.1155/2013/675391.
- Deborah Quaglio; Silvia Corradi; Silvia Erazo; Valeria Vergine; Simone Berardozzi; Fabio Sciubba; Floriana Cappiello; Maria Elisa Crestoni; Fiorentina Ascenzioni; Francesco Imperi; et al.Franco Delle MonacheMattia MoriMaria Rosa LoffredoFrancesca GhirgaBruno CasciaroBruno BottaMaria Luisa Mangoni Structural Elucidation and Antimicrobial Characterization of Novel Diterpenoids from Fabiana densa var. ramulosa. ACS Medicinal Chemistry Letters 2020, 11, 760-765, 10.1021/acsmedchemlett.9b00605.
- Ramona Barbieri; Erika Coppo; A. Marchese; Maria Daglia; Eduardo Sobarzo-Sánchez; Seyed Fazel Nabavi; Seyed Mohammad Nabavi; Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiological Research 2017, 196, 44-68, 10.1016/j.micres.2016.12.003.
- Dianella Savoia; Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiology 2012, 7, 979-990, 10.2217/fmb.12.68.
- Francesca Ghirga; Roberta Stefanelli; Luca Cavinato; Alessandra Lo Sciuto; Silvia Corradi; Deborah Quaglio; Andrea Calcaterra; Bruno Casciaro; Maria Rosa Loffredo; Floriana Cappiello; et al.Patrizia MorelliAlberto AntonelliGian Maria RossoliniMarialuisa MangoniCarmine ManconeBruno BottaMattia MoriFiorentina AscenzioniFrancesco Imperi A novel colistin adjuvant identified by virtual screening for ArnT inhibitors. Journal of Antimicrobial Chemotherapy 2020, -, -, 10.1093/jac/dkaa200.
- Floriana Cappiello; Maria Rosa Loffredo; Cristina Del Plato; Silvia Cammarone; Bruno Casciaro; Deborah Quaglio; Maria Luisa Mangoni; Bruno Botta; Francesca Ghirga; The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics 2020, 9, 325, 10.3390/antibiotics9060325.
- Simone Berardozzi; Flavia Bernardi; Paola Infante; Cinzia Ingallina; Sara Toscano; Elisa De Paolis; Romina Alfonsi; Miriam Caimano; Bruno Botta; Mattia Mori; et al.Lucia Di MarcotullioFrancesca Ghirga Synergistic inhibition of the Hedgehog pathway by newly designed Smo and Gli antagonists bearing the isoflavone scaffold. European Journal of Medicinal Chemistry 2018, 156, 554-562, 10.1016/j.ejmech.2018.07.017.
- Deborah Quaglio; Nadezda Zhdanovskaya; Gloria Tobajas; Viviana Cuartas; Silvia Balducci; Michael S. Christodoulou; Giancarlo Fabrizi; Marta Gargantilla; Eva-María Priego; Álvaro Carmona Pestaña; et al.Daniele PassarellaIsabella ScrepantiBruno BottaRocco PalermoMattia MoriFrancesca GhirgaMaría Jesús Pérez-PérezDanielle Passarella Chalcones and Chalcone-mimetic Derivatives as Notch Inhibitors in a Model of T-cell Acute Lymphoblastic Leukemia. ACS Medicinal Chemistry Letters 2019, 10, 639-643, 10.1021/acsmedchemlett.8b00608.
- Ludovica Lospinoso Severini; Deborah Quaglio; Irene Basili; Francesca Ghirga; Francesca Bufalieri; Miriam Caimano; Silvia Balducci; Marta Moretti; Isabella Romeo; Elena Loricchio; et al.Marella MaroderBruno BottaMattia MoriPaola InfanteLucia Di MarcotullioLospinoso SeveriniMoriDi Marcotullio A Smo/Gli Multitarget Hedgehog Pathway Inhibitor Impairs Tumor Growth. Cancers 2019, 11, 1518, 10.3390/cancers11101518.
- Evans, W.C.; Evans, D.. Chapter 26-Alkaloids; Saunders: Amsterdam, The Netherlands, 2009; pp. 353–415.
- V. M. Dembitsky; Astonishing diversity of natural surfactants: 6. Biologically active marine and terrestrial alkaloid glycosides.. Lipids 2005, 40, 1081-105, 10.1007/s11745-005-1473-2.
- Leen Othman; Ahmad Sleiman; Roula M. Abdel-Massih; Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Frontiers in Microbiology 2019, 10, 911, 10.3389/fmicb.2019.00911.
- Tyler, V.E.; Speedie, M.K.; Robbers, J.E.. Pharmacognosy and Pharmacobiotechnology; Williams & Wilkins: Philadelphia, PA, USA, 1996; pp. 144–185.
- Bikash Debnath; Waikhom Somraj Singh; Manik Das; Sanchari Goswami; Mahesh Kumar Singh; Debasish Maiti; Kuntal Manna; Role of plant alkaloids on human health: A review of biological activities. Materials Today Chemistry 2018, 9, 56-72, 10.1016/j.mtchem.2018.05.001.
- T.P. Tim Cushnie; Andrew J. Lamb; Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents 2005, 26, 343-356, 10.1016/j.ijantimicag.2005.09.002.
- V Amirkia; M Heinrich; Alkaloids as drug leads – A predictive structural and biodiversity-based analysis. Planta Medica 2014, 80, -, 10.1055/s-0034-1394814.
- Amin Thawabteh; Salma Juma; Mariam Bader; Donia Karaman; Laura Scrano; Sabino A. Bufo; Rafik Karaman; The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656, 10.3390/toxins11110656.
- Bahman Khameneh; Milad Iranshahy; Morteza Ghandadi; Davod Ghoochi Atashbeyk; Bibi Sedigheh Fazly Bazzaz; Mehrdad Iranshahi; Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistantStaphylococcus aureus. Drug Development and Industrial Pharmacy 2014, 41, 989-994, 10.3109/03639045.2014.920025.
- Francesca Ghirga; Deborah Quaglio; Patrizio Ghirga; Simone Berardozzi; Giovanni Zappia; Bruno Botta; Mattia Mori; Ilaria D’Acquarica; Occurrence of Enantioselectivity in Nature: The Case of (S )-Norcoclaurine. Chirality 2016, 28, 169-180, 10.1002/chir.22566.
- Cinzia Ingallina; Ilaria D’Acquarica; Giuliano Delle Monache; Francesca Ghirga; Deborah Quaglio; Patrizio Ghirga; Simone Berardozzi; Violeta Markovic; Bruno Botta; The Pictet-Spengler Reaction Still on Stage. Current Pharmaceutical Design 2016, 22, 1808-1850, 10.2174/1381612822666151231100247.
- Menendez, P.; D’Acquarica, I.; Monache, G.D.; Ghirga, F.; Calcaterra, A.; Barba, M.; Macone, A.; Boffi, A.; Bonamore, A.; Botta, B. . Production of Bioactives Compounds: The Importance of Pictet–Spengler Reaction in the XXI Century. In Plant Bioactives and Drug Discovery: Principles, Practice, and Perspectives; John Wiley & Sons, Inc. : New York, NY, USA, 2012; pp. -.
- Andrea Calcaterra; Laura Mangiardi; Giuliano Delle Monache; Deborah Quaglio; Silvia Balducci; Simone Berardozzi; Antonia Iazzetti; Roberta Franzini; Bruno Botta; Francesca Ghirga; et al. The Pictet-Spengler Reaction Updates Its Habits. Molecules 2020, 25, 414, 10.3390/molecules25020414.
- Deborah Quaglio; Giovanni Zappia; Elisa De Paolis; Silvia Balducci; Bruno Botta; Francesca Ghirga; Olefin Metathesis Reaction as a Locking Tool for Macrocycles and Mechanomolecules Construction. Organic Chemistry Frontiers 2018, 5, 3022–3055, 10.1039/c8qo00728d.
- Lekkala Ravindar; Revathi Lekkala; K. P. Rakesh; Abdullah M. Asiri; Hadi M. Marwani; Huali Qin; Carbonyl–olefin metathesis: a key review. Organic Chemistry Frontiers 2018, 5, 1381-1391, 10.1039/c7qo01037k.
- Jehrod B. Brenneman; Stephen F. Martin; Ring-Closing Metathesis as a Construct for the Synthesis of Polycyclic Alkaloids. ChemInform 2006, 9, 1535–1549, 10.1002/chin.200601245.
- Jehrod B. Brenneman; Stephen F. Martin; Ring-Closing Metathesis as a Construct for the Synthesis of Polycyclic Alkaloids. ChemInform 2006, 9, 1535–1549, 10.1002/chin.200601245.