The periosteum, with its outer fibrous and inner cambium layer, lies in a dynamic environment with a niche of pluripotent stem cells for their reparative needs. The inner cambium layer is rich in mesenchymal progenitors, osteogenic progenitors, osteoblasts, and fibroblasts in a scant collagen matrix environment. Their role in union and remodeling of fracture is well known. From a therapeutic standpoint, the periosteum as a source of tissue engineering has gained much attraction.
- periosteum
- mesenchymal stromal cells
- chondrogenesis
- osteogenesis
1. Introduction
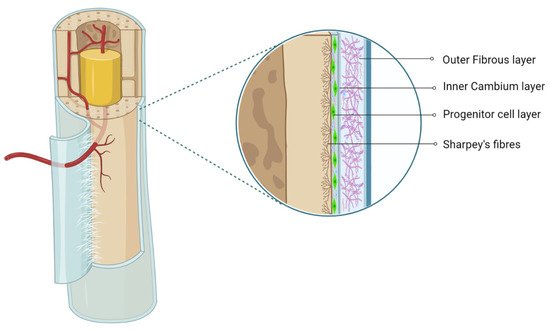
2. Periosteum as MSC Source
The cambium layer of the periosteum contains mesenchymal progenitor cells which can be extrapolated as periosteum-derived MSCs (P-MSCs) [7,8,9][7][8][9]. Various studies have stated that the periosteum is the best cellular therapeutic agent for bone regeneration due to the multipotent nature at the single-cell level, higher proliferation and differentiation rate, and retention of differentiation ability in vitro and in vivo [10,11][10][11]. P-MSCs from load-bearing sites possess more osteogenic potential than flat bones [12]. In cases of fractures, quiescent P-MSCs induce chondrogenesis and osteogenesis, which helps in long-term integration with native bone [13,14][13][14].
3. Characterization and Isolation of P-MSCs
54. Osteogenicity of P-MSCs
P-MSCs sorted with CD-90+ demonstrate higher osteogenic potential than unsorted P-MSCs whether in vitro or in vivo [58][34]. Hence, CD-90+ sorted P-MSCs represent the ideal cell source with higher osteogenic potential for bone regeneration. Upregulation of fibroblast growth factor (FGF)-2, -5, and -6 leads to early callus formation and maintains periosteal osteogenesis [59][35]. Upregulation of FGFR-1 and -2 was observed in proliferating periosteal mesenchyme [59][35]. Upregulated expression of Jag-1 and Notch-2 genes was observed in MSCs of the healing periosteum [60][36]. The lineage differentiation potential of MSCs is mainly governed by PPARγ and Runx2 genes. Runx2 is the key regulator for pro-osteogenesis, whereas PPARγ is responsible for anti-osteoblastogenic effects [61][37]. Runx-2 and -3 are responsible for osteogenesis and chondrogenesis of MSCs. Along with Runx-2, TGF-β1, BMP, Wnt, Hedgehog (HH), and (Nel)-like protein type 1 (NELL-1) activate and regulate osteogenic responses, as shown in Figure 2 and Figure 3 [62,63][38][39].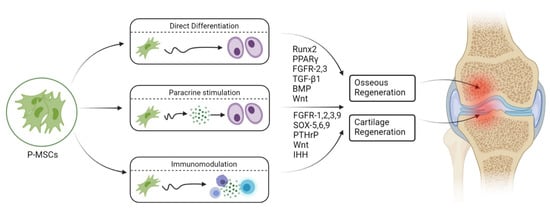
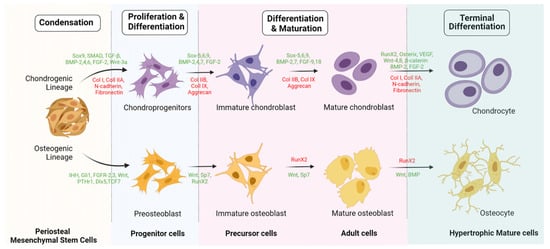
65. Engineered Osteogenesis by P-MSCs
The key challenge in tissue engineering is the formation of new blood vessels (neovasculogenesis) at the transplanted site. Van Gastel et al. observed increased proangiogenic potential when murine P-MSCs were cotransplanted with a collagen calcium phosphate scaffold and endothelial cells in vivo [76][53]. As a result, they exhibited pericyte-like cells which induced hematopoietic stroma with neovasculogenesis [76][53]. Zheng et al. observed enhanced osteogenesis in the form of increased expression of osteocalcin, osteonectin, and type 1 collagen in 3D culture when P-MSCs were loaded onto a poly(lactic-co-glycolic acid) scaffold with allogenic serum [77][54]. Enzymatically harvested and culture-expanded periosteal cells from human rib periosteum exhibited in vitro osteogenesis and chondrogenesis [78][55].86. Chondrogenicity of P-MSCs
Ito et al. studied the chondrocyte precursors in the periosteum, and their observations were as follows: (a) the cambium layer of the periosteum caters to chondrocyte precursors, (b) appositional neochondrogenesis displaces the fibrous layer away from already formed cartilaginous tissue, and (c) chondrogenesis commences from the juxtaosseous to juxtafibrous region of the cambium layer [14]. Periosteal progenitors possess chondrogenic potential in the presence of chondrogenic-dependent transcriptional factors and signaling pathways. In the presence of TGF-β3, periosteal progenitors differentiate into chondrocytes along with atelocollagen, which was further evaluated by immunohistochemical staining for type 2 collagen [109][56].97. Engineered Chondrogenesis of P-MSCs
Various studies have emphasized that the periosteum predominantly gives rise to chondrocytes [115,116][57][58]. Brittberg et al. demonstrated that the periosteum possessed a higher degree of clonogenicity when chondrocytes were cocultured with periosteal cells and agarose [116][58]. Such cocultured chondrocytes expressed higher levels of IL-6 and -8, TGF-β3, and GM-CSF. They concluded that TGF-β3 can promote periosteal chondrogenesis. P-MSC chondrogenesis and MSC differentiation were observed when TGF-β3 was injected subperiosteally [117,118,119][59][60][61]. Periosteal cells from adults and the elderly population retain proliferation and multilineage differentiation capacity. Subperiosteal injection of TGF-β1 and IGF-1 increased the cellular count and phenotypic stability in the cambium layer of periosteum in aged rabbits, as well as in vitro cartilage regeneration [117][59]. In vitro cultured rabbit periosteal explants exhibited chondrogenic potential when exposed to TGF-β1 [120][62]. Cultured chondrocytes showed increased expression of type 2 collagen. Digoxin and ATP increase collagen content in neocartilage by around 110%. The tensile strength of newly formed cartilage was increased by digoxin (280%) and ATP (180%). Digoxin and ATP increased Ca2+ oscillations in monolayer cultured chondrocytes. Hence, calcium modulators enhance neochondrogenesis [121][63]. Evidence has shown that the supplementation of ECM materials such as COMP and aggrecan in a biogel formulation (low-melting-point agarose) mimicked the composition of the cartilage tissue when injected in between bone and the periosteum of the superomedial aspect of rabbit tibia. Regenerated subperiosteal chondrogenic tissue was analyzed for glycosaminoglycan and DNA content, as well as ALP activity. ALP activity was grossly decreased with an increase in GAG and DNA content in regenerated cartilage. This model created a suppressive environment for the chondrocyte hypertrophy niche with chondrogenic progenitors for cartilage tissue engineering in in vitro bioreactors [122][64]. Hence, this milieu provides a broad-based platform for cartilage grafting in cartilage defects.
References
- Dwek, J.R. The periosteum: What is it, where is it, and what mimics it in its absence? Skelet. Radiol. 2010, 39, 319–323.
- Alencar, C.H.M.F.; Silveira, C.R.S.; Cavalcante, M.M.; Vieira, C.G.M.; Teixeira, M.J.D.; Neto, F.A.; de Abreu, A.; Chhabra, A. Periosteum: An imaging review. Eur. J. Radiol. Open 2020, 7, 100249.
- Egawa, S.; Miura, S.; Yokoyama, H.; Endo, T.; Tamura, K. Growth and differentiation of a long bone in limb development, repair and regeneration. Dev. Growth Differ. 2014, 56, 410–424.
- Evans, S.F.; Chang, H.; Knothe Tate, M.L. Elucidating multiscale periosteal mechanobiology: A key to unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng. Part B Rev. 2013, 19, 147–159.
- Mahajan, A. Periosteum: A Highly Underrated Tool in Dentistry. Int. J. Dent. 2011, 2012, e717816.
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277.
- Hutmacher, D.W.; Sittinger, M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003, 9 (Suppl. 1), 45–64.
- Warnke, P.H.; Douglas, T.; Sivananthan, S.; Wiltfang, J.; Springer, I.; Becker, S.T. Tissue engineering of periosteal cell membranes in vitro. Clin. Oral Implants Res. 2009, 20, 761–766.
- Arnsdorf, E.J.; Jones, L.M.; Carter, D.R.; Jacobs, C.R. The periosteum as a cellular source for functional tissue engineering. Tissue Eng. Part A 2009, 15, 2637–2642.
- Colnot, C.; Zhang, X.; Knothe Tate, M.L. Current insights on the regenerative potential of the periosteum: Molecular, cellular, and endogenous engineering approaches. J. Orthop. Res. 2012, 30, 1869–1878.
- Chang, H.; Knothe Tate, M.L. Concise review: The periosteum: Tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl. Med. 2012, 1, 480–491.
- Moore, E.R.; Zhu, Y.X.; Ryu, H.S.; Jacobs, C.R. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res. Ther. 2018, 9, 190.
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum. J. Dent. Res. 2014, 93, 109–116.
- Ito, Y.; Fitzsimmons, J.S.; Sanyal, A.; Mello, M.A.; Mukherjee, N.; O’Driscoll, S.W. Localization of chondrocyte precursors in periosteum. Osteoarthr. Cartil. 2001, 9, 215–223.
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219.
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143.
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 761–766.
- Maleki, M.; Ghanbarvand, F.; Reza Behvarz, M.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126.
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419.
- Lin, C.-S.; Xin, Z.-C.; Dai, J.; Lue, T.F. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol. Histopathol. 2013, 28, 1109–1116.
- Olivares-Navarrete, R.; Rodil, S.E.; Hyzy, S.L.; Dunn, G.R.; Almaguer-Flores, A.; Schwartz, Z.; Boyan, B.D. Role of Integrin Subunits in Mesenchymal Stem Cell Differentiation and Osteoblast Maturation on Graphitic Carbon-coated Microstructured Surfaces. Biomaterials 2015, 51, 69–79.
- Krebsbach, P.H.; Villa-Diaz, L.G. The Role of Integrin α6 (CD49f) in Stem Cells: More than a Conserved Biomarker. Stem Cells Dev. 2017, 26, 1090–1099.
- Ren, G.; Roberts, A.I.; Shi, Y. Adhesion molecules: Key players in Mesenchymal stem cell-mediated immunosuppression. Cell Adhes. Migr. 2011, 5, 20–22.
- Benvenuto, F.; Voci, A.; Carminati, E.; Gualandi, F.; Mancardi, G.; Uccelli, A.; Vergani, L. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res. Ther. 2015, 6, 245.
- Machado, C.D.V.; Telles, P.D.D.S.; Nascimento, I.L.O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. E Hemoter. 2013, 35, 62–67.
- Horwitz, E.M.; Andreef, M.; Frassoni, F. Mesenchymal Stromal Cells. Curr. Opin. Hematol. 2006, 13, 419–425.
- Frey, S.P.; Jansen, H.; Doht, S.; Filgueira, L.; Zellweger, R. Immunohistochemical and molecular characterization of the human periosteum. Sci. World J. 2013, 2013, 341078.
- Yang, H.; Sun, L.; Cai, W.; Gu, J.; Xu, D.; Deb, A.; Duan, J. DDR2, a discoidin domain receptor, is a marker of periosteal osteoblast and osteoblast progenitors. J. Bone Miner. Metab. 2020, 38, 670–677.
- Deveza, L.; Ortinau, L.; Lei, K.; Park, D. Comparative analysis of gene expression identifies distinct molecular signatures of bone marrow- and periosteal-skeletal stem/progenitor cells. PLoS ONE 2018, 13, e0190909.
- Ortinau, L.C.; Wang, H.; Lei, K.; Deveza, L.; Jeong, Y.; Hara, Y.; Grafe, I.; Rosenfeld, S.B.; Lee, D.; Lee, B.; et al. Identification of Functionally Distinct Mx1+αSMA+ Periosteal Skeletal Stem Cells. Cell Stem Cell 2019, 25, 784–796.
- Gao, B.; Deng, R.; Chai, Y.; Chen, H.; Hu, B.; Wang, X.; Zhu, S.; Cao, Y.; Ni, S.; Wan, M.; et al. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J. Clin. Investig. 2019, 129, 2578–2594.
- Groeneveldt, L.C.; Herpelinck, T.; Maréchal, M.; Politis, C.; van IJcken, W.F.J.; Huylebroeck, D.; Geris, L.; Mulugeta, E.; Luyten, F.P. The Bone-Forming Properties of Periosteum-Derived Cells Differ Between Harvest Sites. Front. Cell Dev. Biol. 2020, 8, 554984.
- Olbrich, M.; Rieger, M.; Reinert, S.; Alexander, D. Isolation of Osteoprogenitors from Human Jaw Periosteal Cells: A Comparison of Two Magnetic Separation Methods. PLoS ONE 2012, 7, e47176.
- Kim, Y.-K.; Nakata, H.; Yamamoto, M.; Miyasaka, M.; Kasugai, S.; Kuroda, S. Osteogenic Potential of Mouse Periosteum-Derived Cells Sorted for CD90 In Vitro and In Vivo. Stem Cells Transl. Med. 2016, 5, 227–234.
- Du, X.; Xie, Y.; Xian, C.J.; Chen, L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J. Cell. Physiol. 2012, 227, 3731–3743.
- Dishowitz, M.I.; Terkhorn, S.P.; Bostic, S.A.; Hankenson, K.D. Notch signaling components are upregulated during both endochondral and intramembranous bone regeneration. J. Orthop. Res. 2012, 30, 296–303.
- James, A.W. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica 2013, 2013, e684736.
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792.
- Pratap, J.; Wixted, J.J.; Gaur, T.; Zaidi, S.K.; Dobson, J.; Gokul, K.D.; Hussain, S.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Runx2 transcriptional activation of Indian Hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 2008, 68, 7795–7802.
- Glass, D.A.; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764.
- Takahashi, N.; Maeda, K.; Ishihara, A.; Uehara, S.; Kobayashi, Y. Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Front. Biosci 2011, 16, 21–30.
- Papapoulos, S.E. Targeting sclerostin as potential treatment of osteoporosis. Ann. Rheum. Dis. 2011, 70 (Suppl. 1), i119–i122.
- Kim, J.H.; Liu, X.; Wang, J.; Chen, X.; Zhang, H.; Kim, S.H.; Cui, J.; Li, R.; Zhang, W.; Kong, Y.; et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet. Dis. 2013, 5, 13–31.
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.J.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288.
- Wang, H.; Yin, Y.; Li, W.; Zhao, X.; Yu, Y.; Zhu, J.; Qin, Z.; Wang, Q.; Wang, K.; Lu, W.; et al. Over-expression of PDGFR-β promotes PDGF-induced proliferation, migration, and angiogenesis of EPCs through PI3K/Akt signaling pathway. PLoS ONE 2012, 7, e30503.
- Cheng, T.L.; Cantrill, L.C.; Schindeler, A.; Little, D.G. Induction of periosteal bone formation by intraosseous BMP-2 injection in a mouse model of osteogenesis imperfecta. J. Child. Orthop. 2019, 13, 543–550.
- Seeherman, H.J.; Li, X.J.; Smith, E.; Parkington, J.; Li, R.; Wozney, J.M. Intraosseous injection of rhBMP-2/calcium phosphate matrix improves bone structure and strength in the proximal aspect of the femur in chronic ovariectomized nonhuman primates. J. Bone Joint Surg. 2013, 95, 36–47.
- Shaw, A.T.; Maeda, Y.; Gravallese, E.M. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res. Ther. 2016, 18, 104.
- Claudel, M.; Jouzeau, J.-Y.; Cailotto, F. Secreted Frizzled-related proteins (sFRPs) in osteo-articular diseases: Much more than simple antagonists of Wnt signaling? FEBS J. 2019, 286, 4832–4851.
- Wang, F.-S.; Lin, C.-L.; Chen, Y.-J.; Wang, C.-J.; Yang, K.D.; Huang, Y.-T.; Sun, Y.-C.; Huang, H.-C. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology 2005, 146, 2415–2423.
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. Secreted Frizzled-Related Protein Promotes Bone Regeneration by Human Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2015, 16, 23250–23258.
- Yamauchi, K.; Takahashi, T.; Funaki, K.; Yamashita, Y. Periosteal Expansion Osteogenesis Using Highly Purified Beta-Tricalcium Phosphate Blocks: A Pilot Study in Dogs. J. Periodontol. 2008, 79, 999–1005.
- van Gastel, N.; Torrekens, S.; Roberts, S.J.; Moermans, K.; Schrooten, J.; Carmeliet, P.; Luttun, A.; Luyten, F.P.; Carmeliet, G. Engineering vascularized bone: Osteogenic and proangiogenic potential of murine periosteal cells. Stem Cells 2012, 30, 2460–2471.
- Zheng, Y.; Ringe, J.; Liang, Z.; Loch, A.; Chen, L.; Sittinger, M. Osteogenic potential of human periosteum-derived progenitor cells in PLGA scaffold using allogeneic serum. J. Zhejiang Univ. Sci. B 2006, 7, 817–824.
- Nakahara, H.; Goldberg, V.M.; Caplan, A.I. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J. Orthop. Res. 1991, 9, 465–476.
- Choi, Y.-S.; Lim, S.-M.; Shin, H.-C.; Lee, C.-W.; Kim, S.-L.; Kim, D.-I. Chondrogenesis of human periosteum-derived progenitor cells in atelocollagen. Biotechnol. Lett. 2007, 29, 323–329.
- Gooding, C.R.; Bartlett, W.; Bentley, G.; Skinner, J.A.; Carrington, R.; Flanagan, A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee 2006, 13, 203–210.
- Brittberg, M.; Sjögren-Jansson, E.; Thornemo, M.; Faber, B.; Tarkowski, A.; Peterson, L.; Lindahl, A. Clonal growth of human articular cartilage and the functional role of the periosteum in chondrogenesis. Osteoarthr. Cartil. 2005, 13, 146–153.
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001, 44, 85–95.
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434.
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215.
- O’Driscoll, S.W.; Recklies, A.D.; Poole, A.R. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. JBJS 1994, 76, 1042–1051.
- Makris, E.A.; Huang, B.J.; Hu, J.C.; Chen-Izu, Y.; Athanasiou, K.A. Digoxin and adenosine triphosphate enhance the functional properties of tissue-engineered cartilage. Tissue Eng. Part A 2015, 21, 884–894.
- Caron, M.M.J.; Janssen, M.P.F.; Peeters, L.; Haudenschild, D.R.; Cremers, A.; Surtel, D.A.M.; van Rhijn, L.W.; Emans, P.J.; Welting, T.J.M. Aggrecan and COMP Improve Periosteal Chondrogenesis by Delaying Chondrocyte Hypertrophic Maturation. Front. Bioeng. Biotechnol. 2020, 8, 1036.
