TIGIT is a negative regulator of immune response known to bind to PVR ligands with greater affinity and outcompete the costimulatory receptors, CD226 and CD96, expressed on T cells, thereby inhibiting the activation, proliferation, and differentiation of T cells (). Further, TIGIT engagement ensures the survival of inhibited T cells by activating cell survival pathways.. TIGIT activation on NK cells was shown to inhibit cytotoxic granule polarization and IFN-γ production and decrease NK cell cytotoxicity. In addition, TIGIT interaction on Tregs skews the cytokine balance, suppresses Th1 or Th17 phenotype, and induces Th2 phenotype.
- TIGIT
- immune checkpoints
- immunotherapy and cancer
1. Introduction
The idea of using immune response against abnormal cells in the body to treat cancer has been tested in the past few decades and evolved from using recombinant cytokines to adoptive cell transfer [1,2][1][2]. The first generation of immunotherapies like high-dose interleukin-2 were limited by low response rates and high incidence of serious adverse events, but the durability of response encouraged further research in the field [3,4,5][3][4][5]. Discovery of checkpoints of T-cell activation and development of monoclonal antibodies targeting the checkpoints dramatically changed the outcomes of immunotherapy [6,7,8,9,10,11,12][6][7][8][9][10][11][12]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) were the early targets that were discovered and characterized in the late 1980s and early 1990s, respectively [13,14,15,16,17,18,19][13][14][15][16][17][18][19]. Both CTLA-4 and PD-1 have been shown to be reliable targets, and to date, seven drugs have been approved for different types of cancers, such as melanoma and lung cancer [20,21,22,23,24][20][21][22][23][24]. In addition to monotherapy, combination of CTLA-4 and PD-1 blockers is also approved for treatment of multiple cancer types [23]. While the CTLA-4 and PD-1 blockers had decent and durable response rates, a large fraction of patients did not respond to the treatment, and the incidence of serious adverse events was high in the responding patients [25,26,27][25][26][27]. The need for safer targets that can be blocked or activated to achieve reasonable anti-tumor response with manageable adverse events and that can be combined with PD-1/PD-L1 blockers or other immune checkpoint blockers led to the identification of T-cell immunoglobulin and ITIM domain (TIGIT), an inhibitory immune checkpoint, and the development of anti-TIGIT antibodies.
TIGIT is considered as an important target mainly because of its expression profile (natural killer cells (NK cells), cytotoxic CD8 + T cells and regulatory T cells (Tregs) [28]. More importantly, the phenotype of Tigit −/− mouse was reported to be mild, and the knockout mice did not spontaneously develop autoimmunity, indicating a comparatively milder safety profile [29].
2. Tigit
TIGIT expression is mainly seen on resting CD4 + CD25 hi Treg cells, activated T cells, NK cells, NKT cells, and memory T cells. Naïve CD4 + T cells do not express TIGIT, but its expression is induced at mRNA levels upon activation [35][30]. TIGIT has been reported as marker for CD8+ T-cell exhaustion and is also a characteristic marker for Tregs in the tumor microenvironment [7,28,29,37,38][7][28][29][31][32].
CD155 expression is mainly reported on DCs, T cells, B cells, and macrophages, whereas CD112 is widely expressed on both hematopoietic and non-haematopoietic tissues, including bone marrow, lung, pancreas, and kidney [40,41][33][34]. CD113 expression is limited to non-hematopoietic tissues, such as lung, liver, testis, kidney, and placenta [42][35]. Several human cancers are reported to overexpress CD155 and CD112 [43,44,45][36][37][38]. Interestingly, interferon-γ (IFN- γ) was shown to up-regulate the expression of CD155 on human vascular endothelial cells, a mechanism similar to induction of PD-1/PD-L1 pathway [46][39].
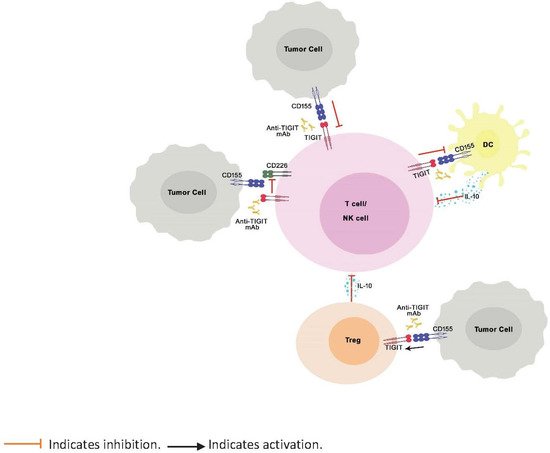
TIGIT is a negative regulator of immune response known to bind to PVR ligands with greater affinity and outcompete the costimulatory receptors, CD226 and CD96, expressed on T cells, thereby inhibiting the activation, proliferation, and differentiation of T cells ( Figure 1 ). Further, TIGIT engagement ensures the survival of inhibited T cells by activating cell survival pathways. [7]. TIGIT activation on NK cells was shown to inhibit cytotoxic granule polarization and IFN-γ production and decrease NK cell cytotoxicity [30,47][40][41]. In addition, TIGIT interaction on Tregs skews the cytokine balance, suppresses Th1 or Th17 phenotype, and induces Th2 phenotype [29,48][29][42]. However, unlike CTLA-4 and PD-1, which, when knocked out in mice, are known to manifest as severe and spontaneous autoimmune phenotype [17,49,50,51][17][43][44][45], TIGIT knock-out mice do not spontaneously develop autoimmune phenotype, indicating mild to moderate control of TIGIT over immune response [29].
Even before the discovery of TIGIT, its ligands were known to be upregulated on the surface of tumor cell surface. Expression of nectin family of proteins and their role in cell adhesion and survival was reported in tumors from epithelial origin, such as non-small cell lung cancer, colon cancer, and metastatic neuroblastoma, and also tumors from hematopoietic origin, such as myeloid leukemia [43,52,53,54,55][36][46][47][48][49]. High expression of CD155 was shown to be an independent prognostic marker and predictor of poor clinical outcome in breast cancer patients [56][50]. The recently discovered ligand for TIGIT, nectin 4, was shown to be overexpressed in breast, bladder, lung, and pancreatic cancers [57][51]. On the other hand, TIGIT expression was also reported to be upregulated on lymphocytes in tumor microenvironment. Studies showed TIGIT expression on CD8 + , CD4 + T cells, and NK cells paralleled to that of PD-1 in hepatocellular, lung, and colorectal cancers and in Hodgkin’s lymphoma [58,59,60,61,62,63,64,65][52][53][54][55][56][57][58][59].
3. Anti-Tigit Antibodies in Development
| Generic Name | Type | FcγR Status | Company | Status |
|---|
| Tiragolumab (MTIG7192A) | Fully human IgG1 | Active | Genentech | Phase III |
| Ociperlimab (BGB-A1217) | Humanized IgG1 | Active | BeiGene USA, Inc | Phase III |
| Vibostolimab (MK-7684) | Fully human IgG1 | Active | Merck & Co Inc | Phase II |
| Domvanalimab (AB-154) | Fully human IgG1 | Inactive | Arcus Biosciences Inc | Phase II |
3.2. Origin
3.3. IgG Isotype and FcγR Binding
3.4. Dose
| Drug | Phase | Dose and Regimen | Comment | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tiragolumab | Phase III Multiple Solid tumors |
2 mg to 1200 mg Q3W RP2D: 600 mg Q3W |
100% receptor occupancy seen at ≥30 mg and clinical activity observed at doses 400 mg to 600 mg. 600 mg Q3W was proposed as dose for Ph2 study. |
Bendell et al. AACR 2020 | |||||
| Ociperlimab | Phase III | 50 mg to 900 mg RP2D: 900 mg Q3W |
100% receptor occupancy was observed at 50 mg, and linear PK was observed through 900 mg. | NCT04746924 | |||||
| Domvanalimab (AB-154) | Phase I NSCLC |
0.5 mg/kg; 1 mg/kg & 3 mg/kg Q2W | 100% receptor occupancy seen at 3 mg/kg | Anderson et al. SITC 2019 p260 | |||||
| Vibostolimab (MK-7684) | Phase I Multiple Solid tumors |
2.1 mg to 700 mg Q3W RP2D: 200 mg Q3W |
ORR 19% in combination with pembrolizumab Vibostolimab well tolerated as monotherapy and in combination with 200 mg pembrolizumab |
Golan et al. SITC 2018 | |||||
| BMS-986207 | Fully human IgG1 | Inactive | Bristol-Myers Squibb Co | Phase II | |||||
| BMS-986207 | Phase I/II | Not disclosed | No details | NCT02913313 | EOS-448 | ||||
| EOS-448 | Fully human IgG1 | Active | iTeos Therapeutics SA | Phase II | |||||
| Phase I | 0.1 mg/kg, 1 mg/kg and 10 mg/kg | Receptor occupancy increased with dose. Nearly 100% occupancy was seen at 10 mg/kg dose. Dose-limiting toxicity was not seen. | Nguyen et al. AACR 2020 | ASP-8374 | Fully human IgG4 | Inactive | Astellas Pharma Inc | Phase I | |
| ASP-8374 | Phase I Solid tumors |
Not disclosed | Details not available | NCT03260322 | COM-902 | Mouse/cyno cross-reactive fully human IgG1 antibody | NA | Compugen Ltd. | |
| COM-902 | Phase I Solid tumors | Phase I | |||||||
| 7 doses to be tested for dose limiting toxicity. | Q3W regimen | Data not available. Study posted in April 2020. | NCT04354246 | Etigilimab | Fully human IgG1 | Active | Mereo Biopharma Group Plc | Phase I | |
| Etigilimab | Phase I | 0.3 mg/kg to 20 mg/kg Q2W | Safely administered up to 20 mg/kg. Stable disease was seen in 7/18 patients across all doses | IBI-939 | NA | NA | Innovent Biologics Inc | IND Filed | |
| Sharma et al. SITC 2018 | AGEN-1307 | Fully human IgG1 | Active and enhanced | Agenus Inc | Preclinical | ||||
| CASC-674 | Fully human IgG2a | Inactive | Seattle Genetics Inc | Preclinical | |||||
| Anti-PVR Antibody (NB-6253) | NA | NA | Northern Biologics Inc | Preclinical | |||||
| PH-804 | NA | NA | Phio Pharmaceuticals Corp | Preclinical | |||||
| TIGIT-PD-L1 dual | NA | NA | Aurigene Discovery Technologies Ltd. | Preclinical |
3.1. Factors Considered during Development
3.5. Safety
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97.
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668.
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458.
- Rotte, A.; Bhandaru, M. Interleukin-2. In Immunotherapy of Melanoma; Springer International Publishing: Cham, Switzerland, 2016.
- Rotte, A.; Bhandaru, M. Interferon-a2b. In Immunotherapy of Melanoma; Springer International Publishing: Cham, Switzerland, 2016.
- Bhandaru, M.; Rotte, A. Blockade of programmed cell death protein-1 pathway for the treatment of melanoma. J. Dermatol. Res. Ther. 2017, 1, 1–11.
- Bhandaru, M.; Rotte, A. Monoclonal Antibodies for the Treatment of Melanoma: Present and Future Strategies. Methods Mol. Biol. 2019, 1904, 83–108.
- Rotte, A.; Bhandaru, M.; Zhou, Y.; McElwee, K.J. Immunotherapy of melanoma: Present options and future promises. Cancer Metastasis Rev. 2015, 34, 115–128.
- Varade, J.; Magadan, S.; Gonzalez-Fernandez, A. Human immunology and immunotherapy: Main achievements and challenges. Cell Mol. Immunol. 2020.
- Zhao, D.; Xie, B.; Yang, Y.; Yan, P.; Liang, S.N.; Lin, Q. Progress in immunotherapy for small cell lung cancer. World J. Clin. Oncol. 2020, 11, 370–377.
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily—CTLA-4. Nature 1987, 328, 267–270.
- Stamper, C.C.; Zhang, Y.; Tobin, J.F.; Erbe, D.V.; Ikemizu, S.; Davis, S.J.; Stahl, M.L.; Seehra, J.; Somers, W.S.; Mosyak, L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 2001, 410, 608–611.
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465.
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895.
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151.
- Shinohara, T.; Taniwaki, M.; Ishida, Y.; Kawaichi, M.; Honjo, T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics 1994, 23, 704–706.
- Nishimura, H.; Honjo, T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001, 22, 265–268.
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182.
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39.
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106.
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255.
- Lemaire, V.; Shemesh, C.; Rotte, A. Pharmacology-Based Ranking of Anti-Cancer Drugs to Guide Clinical Development of Cancer Immunotherapy Combinations; 2021.
- Tarhini, A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: The underlying mechanisms and clinical management. Scientifica 2013, 2013, 857519.
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148.
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2016, 27, 559–574.
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8.
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.F.; Joller, N.; Tan, D.J.; Teng, M.W.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 2015, 125, 4053–4062.
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57.
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014, 26, 923–937.
- Chew, G.M.; Fujita, T.; Webb, G.M.; Burwitz, B.J.; Wu, H.L.; Reed, J.S.; Hammond, K.B.; Clayton, K.L.; Ishii, N.; Abdel-Mohsen, M.; et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016, 12, e1005349.
- Eberle, F.; Dubreuil, P.; Mattei, M.G.; Devilard, E.; Lopez, M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 1995, 159, 267–272.
- Lopez, M.; Aoubala, M.; Jordier, F.; Isnardon, D.; Gomez, S.; Dubreuil, P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood 1998, 92, 4602–4611.
- Satoh-Horikawa, K.; Nakanishi, H.; Takahashi, K.; Miyahara, M.; Nishimura, M.; Tachibana, K.; Mizoguchi, A.; Takai, Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 2000, 275, 10291–10299.
- Masson, D.; Jarry, A.; Baury, B.; Blanchardie, P.; Laboisse, C.; Lustenberger, P.; Denis, M.G. Overexpression of the CD155 gene in human colorectal carcinoma. Gut 2001, 49, 236–240.
- Bevelacqua, V.; Bevelacqua, Y.; Candido, S.; Skarmoutsou, E.; Amoroso, A.; Guarneri, C.; Strazzanti, A.; Gangemi, P.; Mazzarino, M.C.; D’Amico, F.; et al. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget 2012, 3, 882–892.
- Oshima, T.; Sato, S.; Kato, J.; Ito, Y.; Watanabe, T.; Tsuji, I.; Hori, A.; Kurokawa, T.; Kokubo, T. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol. Cancer 2013, 12, 60.
- Escalante, N.K.; von Rossum, A.; Lee, M.; Choy, J.C. CD155 on human vascular endothelial cells attenuates the acquisition of effector functions in CD8 T cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1177–1184.
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004.
- Zhang, B.; Zhao, W.; Li, H.; Chen, Y.; Tian, H.; Li, L.; Zhang, L.; Gao, C.; Zheng, J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016, 65, 305–314.
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581.
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547.
- Chambers, C.A.; Sullivan, T.J.; Allison, J.P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 1997, 7, 885–895.
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322.
- Irie, K.; Shimizu, K.; Sakisaka, T.; Ikeda, W.; Takai, Y. Roles and modes of action of nectins in cell-cell adhesion. Semin. Cell Dev. Biol. 2004, 15, 643–656.
- Sakisaka, T.; Takai, Y. Biology and pathology of nectins and nectin-like molecules. Curr. Opin. Cell Biol. 2004, 16, 513–521.
- Irie, K.; Shimizu, K.; Sakisaka, T.; Ikeda, W.; Takai, Y. Roles of nectins in cell adhesion, signaling and polarization. Handb. Exp. Pharmacol. 2004, 343–372.
- Fuchs, A.; Colonna, M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin. Cancer Biol. 2006, 16, 359–366.
- Stamm, H.; Oliveira-Ferrer, L.; Grossjohann, E.M.; Muschhammer, J.; Thaden, V.; Brauneck, F.; Kischel, R.; Muller, V.; Bokemeyer, C.; Fiedler, W.; et al. Targeting the TIGIT-PVR immune checkpoint axis as novel therapeutic option in breast cancer. Oncoimmunology 2019, 8, e1674605.
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013.
- Duan, X.; Liu, J.; Cui, J.; Ma, B.; Zhou, Q.; Yang, X.; Lu, Z.; Du, Y.; Su, C. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma. Mol. Med. Rep. 2019, 20, 3773–3781.
- Hinsch, A.; Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; et al. Expression of the immune checkpoint receptor TIGIT in seminoma. Oncol. Lett. 2019, 18, 1497–1502.
- Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; Debatin, N.F.; et al. Patterns of TIGIT Expression in Lymphatic Tissue, Inflammation, and Cancer. Dis. Markers 2019, 2019, 5160565.
- Li, W.; Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; Debatin, N.F.; et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer 2018, 18, 1209.
- Au, Q.; Hanifi, A.; Parnell, E.; Kuo, J.; Leones, E.; Sahafi, F.; Pham, K.; Padmanabhan, R.K.; Hoe, N.; William, J. Characterization of TIGIT expression using MultiOmyxTM hyperplexed immunofluorescence assay in solid tumors . In Proceedings of the American Association for Cancer Research Annual Meeting. Cancer Res. 2019, 79, AM2019–AM2497.
- Pal, S.K.; Vanderwalde, A.M.; Szeto, C.; Reddy, S.; Hamid, O. PD-L1 expression is strongly associated with TIGIT, FOXP3 and LAG3 across advanced cancers, but not OX40, TIM3 and IDO. Ann. Oncol. 2018, 29, VIII421–VIII422.
- Sun, Y.; Luo, J.; Chen, Y.; Cui, J.; Lei, Y.; Cui, Y.; Jiang, N.; Jiang, W.; Chen, L.; Chen, Y.; et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma). Int. Immunopharmacol. 2020, 80, 106198.
- Yu, H.; Koczara, C.; Lohinai, Z.; Badzio, A.; Czapiewski, P.; Döme, B.; Moldvay, J.; Fillinger, J.; Gao, D.; Ellison, K.; et al. Expression of the Immune Checkpoint Axis-PVR/TIGIT in Small Cell Lung Cancer . J. Thor. Oncol. 2018, 1, S974–S975.
- Roche/Genentech. Roche’s Novel Anti-Tigit Tiragolumab Granted Fda Breakthrough Therapy Designation in Combination with Tecentriq for Pd-L1-High Non-Small Cell Lung Cancer. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0000498.pub2 (accessed on 17 September 2021).
- Ma, L.; Gai, J.; Qiao, P.; Li, Y.; Li, X.; Zhu, M.; Li, G.; Wan, Y. A novel bispecific nanobody with PD-L1/TIGIT dual immune checkpoint blockade. Biochem. Biophys. Res. Commun. 2020, 531, 144–151.
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265.
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 576–588.
- Teillaud, J.L. Antibody-dependent Cellular Cytotoxicity (ADCC). In eLS; Wiley: Hoboken, NJ, USA, 2012.
- Zahavi, D.; AlDeghaither, D.; O’Connell, A.; Weiner, L.M. Enhancing antibody-dependent cell-mediated cytotoxicity: A strategy for improving antibody-based immunotherapy. Antib. Ther. 2018, 1, 7–12.
- Lo Nigro, C.; Macagno, M.; Sangiolo, D.; Bertolaccini, L.; Aglietta, M.; Merlano, M.C. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: Biological evidence and clinical perspectives. Ann. Transl. Med. 2019, 7, 105.
- Temming, A.R.; de Taeye, S.W.; de Graaf, E.L.; de Neef, L.A.; Dekkers, G.; Bruggeman, C.W.; Koers, J.; Ligthart, P.; Nagelkerke, S.Q.; Zimring, J.C.; et al. Functional Attributes of Antibodies, Effector Cells, and Target Cells Affecting NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity. J. Immunol. 2019, 203, 3126–3135.
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.; Quek, J.K.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310.
- Collins, D.M.; O’Donovan, N.; McGowan, P.M.; O’Sullivan, F.; Duffy, M.J.; Crown, J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann. Oncol. 2012, 23, 1788–1795.
- Iannello, A.; Ahmad, A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005, 24, 487–499.
- Waight, J.D.; Chand, D.; Dietrich, S.; Gombos, R.; Horn, T.; Gonzalez, A.M.; Manrique, M.; Swiech, L.; Morin, B.; Brittsan, C.; et al. Selective FcgammaR Co-engagement on APCs Modulates the Activity of Therapeutic Antibodies Targeting T Cell Antigens. Cancer Cell 2018, 33, 1033–1047.e5.
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014, 2, 846–856.
- Lu, S.; Wang, J.; Yu, Y.; Yu, X.; Hu, Y.; Ai, X.; Ma, Z.; Li, X.; Zhuang, W.; Liu, Y.; et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J. Thorac. Oncol. 2021, 38.
- Osarogiagbon, R.U. Tislelizumab-A Promising New Option for Enhancing Chemotherapy Benefit in Treatment for Advanced Squamous Cell Lung Cancer. JAMA Oncol. 2021, 7, 717–719.
- Wang, J.; Lu, S.; Yu, X.; Hu, Y.; Sun, Y.; Wang, Z.; Zhao, J.; Yu, Y.; Hu, C.; Yang, K.; et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 709–717.
- Chand, D.; Waight, J.D.; Paltrinieri, E.; Dietrich, S.; Bushell, M.; Costa, M.; Gombos, R.; Wilson, N.S.; Buell, J.S.; Stein, R.B.; et al. FcgR co-engagement by anti-TIGIT monoclonal antibodies enhances T cell functionality and antitumor immune responses . In Proceedings of the American Association for Cancer Research Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019; p. 2390.
- Anderson, A.E.; Lopez, A.; Udyavar, A.; Narasappa, N.; Lee, S.; DiRenzo, D.; Zhang, K.; Singh, H.; Zhao, S.; Gerrick, K.; et al. Characterization of AB154, a Humanized, Non-Depleting α-TIGIT Antibody Undergoing Clinical Evaluation in Subjects with Advanced Solid Tumors. In Proceedings of the SITC Annual Meeting, National Harbor, MD, USA, 6–10 November 2019.
- Tiragolumab Impresses in Multiple Trials. Cancer Discov. 2020, 10, 1086–1087.
