Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Alessandro Parodi.
Diabetic retinopathy represents a leading cause of vision loss, causing a significant structural and functional impairment of the retinal and choroidal capillary network. Diabetes mellitus is a group of diseases characterized by chronic hyperglycemia, and the most common expressions of this condition are type 1, type 2, and gestational diabetes.
- diabetic retinopathy
- ocular barriers
- nanomedicine
1. Introduction
Diabetes mellitus is a group of diseases characterized by chronic hyperglycemia, and the most common expressions of this condition are type 1, type 2, and gestational diabetes [1]. While type 1 diabetes is an autoimmune disease causing an abnormal immune response against insulin-producing β-cells, significantly blunting the expression of this hormone [2], type 2 [3] and gestational diabetes [4] are also characterized by a certain level of skeletal muscle, liver, and adipose tissue insulin resistance. Genetic, environmental, and behavioral factors, as well as maternal obesity, can cause these conditions. Diabetes incidence is increasing worldwide [5]: the population affected by this disease triplicated in the last 40 years [6], and it keeps on growing [7]. Diabetes-associated conditions affect multiple organs and organ systems, resulting in polyneuropathy [8], angiopathy [9], infections [10], nephropathy [10], dementia [11], cardiovascular complications [12], lower limb amputation [13], and blindness [14]. These phenomena are often irreversible and accompanied by a structural and functional impairment of the tissue microcirculation [15]. Vascular degeneration is particularly prominent in the eye, leading to diabetic retinopathy (DR) [16]. DR affects patients aged between 20 and 65 [17], and its symptoms appear about ten years after diabetes onset [18]. It has been estimated that 20–30 million patients are at risk of irreversible vision loss because of DR [19[19][20],20], which is currently one of the leading causes of visual impairment (Figure 1) [21,22,23][21][22][23] and the leading cause of blindness in preventable retinal diseases [24].
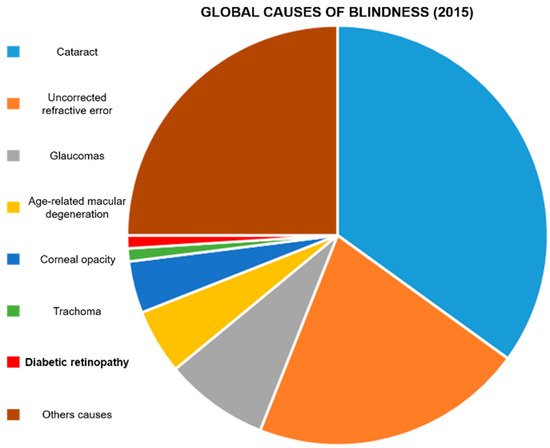
Figure 1. Global causes of blindness: In 2015, it was estimated that DR caused blindness in 400,000 people. Diabetic retinopathy represents 1% of the total blind population. Data were extrapolated from Akland et al. [12].
DR is caused by a significant retinal and choroidal capillary network degeneration, due to a local chronic inflammation sustained by advanced-glycation end-products (AGEs) [25], reactive oxygen species (ROS) [26], growth factors, and interleukins [27,28][27][28].
In DR, the blood–retinal barrier (BRB) becomes highly permeable, causing local edema, necrosis, and ischemic phenomena [29]. The BRB function depends on the integrity and proper conformation of endothelial intercellular junction complexes that are already significantly affected during the first phases of DR. A high vascular endothelial growth factor (VEGF) pathway activity [30] and a misbalance in ROS generation and elimination [31,32,33][31][32][33] induce junction disassembly and leaky blood capillary formation [34]. These phenomena can also affect the protein composition of the vitreous humor [35].
2. Clinical Management of DR
Clinically, DR is classified in non-proliferative and proliferative DR, and in six stages of retinal degeneration, varying from small bleeding events to significant neovascularization and retinal detachment (Figure 2) [38,39,40][38][39][40]. In addition to structural decline, the stress occurring during DR affects photoreceptor cell function and viability [41].
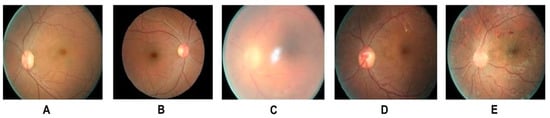
Figure 2. Different stages of DR: (A) Healthy retina (B) mild NPDR (detectable presence of micro aneurysms) (C) moderate NPDR (significant presence of micro aneurysms) (D) severe NPDR (Intraretinal microvascular abnormalities) (E) PDR (presence of hemorrhages). Adapted from Shankar et al. [22].
Optical coherence tomography and fluorescein angiography are commonly used to properly diagnose DR [42], while current therapies for the advanced states of this condition include invasive interventions based on laser photocoagulation (pan-retinal photocoagulation [43]) and vitrectomy [44]. Laser therapy aims at mitigating potential hemorrhagic events by ablating retinal capillary microaneurysms. This procedure requires repetitive applications, and can cause hemorrhages. On the other hand, vitrectomy is a surgical intervention performed to reduce tissue edema and clean the retina from cellular and tissue debris.
Systemic pharmacological treatments [45] aim at lowering blood glucose [46] and lipid [47] levels by adjusting doses and frequency of insulin administration, or administering therapeutics like fenofibrate [48] and statins [49], respectively. In this context also anti-hypertensive therapies [50] showed beneficial properties to mitigate DR progression. However, pharmacological treatments locally administered via subretinal and intravitreal injections are preferred to rapidly and vigorously target the posterior segment of the eye [7].
Typical medications for this disease target the vascular pathologic process and include vascular protective [51] and anti-inflammatory drugs [52[52][53],53], angiogenesis inhibitors [54,55,56,57][54][55][56][57], modulators of the microcirculation [58,59][58][59]. More recently, peroxisome proliferator-activated receptor (PPAR) agonists showed beneficial effects in reducing inflammation, normalizing vascular function, and mitigating ROS-associated damages [60]. These therapies can have a heterogeneous patient response, side effects, and sometimes high associated costs [49,61,62,63,64][49][61][62][63][64].
In addition, when locally inoculated, they still need to overcome the ocular biological barriers, significantly mitigating their retinal targeting properties, without mentioning the discomfort provoked to the patient by these therapies.
Nanomedicine can provide different benefits to improve DR treatments, increasing therapeutic residence time in the eye and providing controlled drug release. This review will examine different nanoplatforms tested for this purpose, focusing on the material properties that make these technologies attractive for DR. In particular, we focused on technologies designed to normalize vascular degeneration in diabetic retinopathy that represent the primary cause of this disease. A few attempts [65,66,67][65][66][67] to develop nanotechnology for reversing and mitigating diabetic retinopthy neurodegeneration were recently performed, but they do not represent the subject of this review. Article inclusion was performed using Google Scholar and PubMed search engines between august and November 2021. A date sorting filter was applied to include papers not older than five years. To select the included articles, we performed literature research using combinations of the following keywords: diabetic retinopathy; ocular barriers; nanomedicine; polymer nanoparticles; albumin nanoparticles; inorganic nanoparticles; extracellular vesicles.
3. Nanomedicine Application in DR
The application of nanomedicine in DR could improve current therapies for this disease. Drug encapsulation in nanostructure can increase drug solubility and retention in the vitreous humor after the injection. In addition, controlled release properties can reduce the number of injections necessary to achieve significant clinical results. Nanocarrier size, surface charge, and shape are fundamental parameters to consider for developing effective drug formulations for intravitreal injections. While a larger size can increase the drug retention time and controlled release, it can negatively impact particle diffusion in the vitreous humor and retinal targeting. Carriers smaller than 500 nm showed a certain degree of diffusion that was inhibited when the particles were larger than one micron [80][68]. On the other hand, Koo et al. correlated particle diffusion with their surface charge [70][69]. They synthesized seven groups of polymeric, hybrid delivery platforms with a relatively narrow size range (between 200 and 350 nm) and very different surface charges (between -25 and +30 mV). The scientist discovered that a strong positive charge inhibited particle (polyethyleneimine-PEI) diffusion in the vitreous humor, probably due to ionic interactions with the negatively charged proteins of this matrix. On the other hand, a moderate positive charge coupled with antifouling agents (glycol chitosan alone or hybridized with PEI) significantly increased their diffusion in the vitreous humor, and allowed retinal inner limiting membrane targeting. Finally, anionic particles based on albumin could also penetrate the deeper layers of the retina. However, the size of these particles was not compatible with the pore size of the retinal tissue, and more investigation indicated that Muller cells could favor this process, likely through active transport. Moreover, the shape of the particles could represent an essential factor in designing retina-targeted nanocarriers. In particular, Shafaie et al. [80][68] demonstrated that rod-like nanoparticles could efficiently diffuse in the vitreal matrix, but more research on this topic is needed.References
- Center for Disease and Control Prevention. Diabetes. Available online: https://www.cdc.gov/diabetes/basics/diabetes.html (accessed on 11 October 2021).
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462.
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 1–22.
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 1–19.
- Ingelfinger, J.R.; Jarcho, J.A. Increase in the incidence of diabetes and its implications. N. Engl. J. Med. 2017, 376, 1473–1474.
- World Health Organization. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 11 October 2021).
- Liu, Y.; Wu, N. Progress of Nanotechnology in Diabetic Retinopathy Treatment. Int. J. Nanomed. 2021, 16, 1391.
- Metsker, O.; Magoev, K.; Yakovlev, A.; Yanishevskiy, S.; Kopanitsa, G.; Kovalchuk, S.; Krzhizhanovskaya, V.V. Identification of risk factors for patients with diabetes: Diabetic polyneuropathy case study. BMC Med. Inform. Decis. Mak. 2020, 20, 1–15.
- Frolov, D.V.; Kryukov, E.V.; Gerasimenko, M.Y.; Kulikov, A.G. Combined physical therapy for diabetic angiopathy. Russ. J. Physiother. Balneol. Rehabil. 2020, 19, 25–31.
- Unnikrishnan, R.; Misra, A. Infections and diabetes: Risks and mitigation with reference to India. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1889–1894.
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604.
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes/Metab. Res. Rev. 2018, 34, e3047.
- Shatnawi, N.J.; Al-Zoubi, N.A.; Hawamdeh, H.M.; Khader, Y.S.; Garaibeh, K.; Heis, H.A. Predictors of major lower limb amputation in type 2 diabetic patients referred for hospital care with diabetic foot syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 313.
- Maseko, S.; van Staden, D.; Mhlongo, E. The Rising Burden of Diabetes-Related Blindness: A Case for Integration of Primary Eye Care into Primary Health Care in Eswatini. Healthcare 2021, 9, 835.
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T. Determinants of quantitative optical coherence tomography angiography metrics in patients with diabetes. Sci. Rep. 2017, 7, 1–10.
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019, 45, 517–527.
- Alghadyan, A.A. Diabetic retinopathy–An update. Saudi J. Ophthalmol. 2011, 25, 99–111.
- Tarr, J.M.; Kaul, K.; Wolanska, K.; Kohner, E.M.; Chibber, R. Retinopathy in diabetes. Diabetes 2013, 88–106.
- Chopdar, A.; Chakravarthy, U.; Verma, D. Age related macular degeneration. BMJ 2003, 326, 485–488.
- Pollinger, K.; Hennig, R.; Ohlmann, A.; Fuchshofer, R.; Wenzel, R.; Breunig, M.; Tessmar, J.; Tamm, E.R.; Goepferich, A. Ligand-functionalized nanoparticles target endothelial cells in retinal capillaries after systemic application. Proc. Natl. Acad. Sci. USA 2013, 110, 6115–6120.
- Bhagat, N.; Zarbin, M.A. Epidemiology, risk factors, and pathophysiology of diabetic retinopathy. In Clinical Strategies in the Management of Diabetic Retinopathy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19.
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234.
- Ackland, P.; Resnikoff, S.; Bourne, R. World blindness and visual impairment: Despite many successes, the problem is growing. Community Eye Health 2017, 30, 71.
- Mahaling, B.; Srinivasarao, D.A.; Raghu, G.; Kasam, R.K.; Reddy, G.B.; Katti, D.S. A non-invasive nanoparticle mediated delivery of triamcinolone acetonide ameliorates diabetic retinopathy in rats. Nanoscale 2018, 10, 16485–16498.
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717.
- WWu, M.-Y.; Yiang, G.-T.; Lai, T.-T.; Li, C.-J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2018, 2018, 1–12.
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605.
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323.
- Lange, J.; Hadziahmetovic, M.; Zhang, J.; Li, W. Region-specific ischemia, neovascularization and macular oedema in treatment-naïve proliferative diabetic retinopathy. Clin. Exp. Ophthalmol. 2018, 46, 757–766.
- Saravia, M.; Zeman, L.; Ingolotti, M.; Schlaen, A. The VEGF paradox: Does diabetic retinopathy protect from age related macular degeneration? Med. Hypotheses 2017, 109, 156–161.
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163.
- Pan, H.-Z.; Zhang, H.; Chang, D.; Li, H.; Sui, H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br. J. Ophthalmol. 2008, 92, 548–551.
- Yamagishi, S.-i.; Matsui, T. Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr. Pharm. Biotechnol. 2011, 12, 362–368.
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev. Cell 2009, 16, 209–221.
- Ouchi, M.; West, K.; Crabb, J.W.; Kinoshita, S.; Kamei, M. Proteomic analysis of vitreous from diabetic macular edema. Exp. Eye Res. 2005, 81, 176–182.
- Wu, F.; Lamy, R.; Ma, D.; Laotaweerungsawat, S.; Chen, Y.; Zhao, T.; Ma, W.; Zhang, F.; Psaras, C.; Stewart, J.M. Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26.
- Wilkinson, C.; Ferris III, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682.
- Kandhasamy, J.P.; Balamurali, S.; Kadry, S.; Ramasamy, L.K. Diagnosis of diabetic retinopathy using multi level set segmentation algorithm with feature extraction using svm with selective features. Multimed. Tools Appl. 2020, 79, 10581–10596.
- Hwang, H.B.; Jee, D.; Kwon, J.-W. Characteristics of diabetic macular edema patients with serous retinal detachment. Medicine 2019, 98, e18333.
- Shankar, K.; Perumal, E.; Elhoseny, M.; Nguyen, P.T. An iot-cloud based intelligent computer-aided diagnosis of diabetic retinopathy stage classification using deep learning approach. CMC—Comput. Mater. Contin. 2021, 66, 1665–1680.
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358.
- Yao, X.; Alam, M.N.; Le, D.; Toslak, D. Quantitative optical coherence tomography angiography: A review. Exp. Biol. Med. 2020, 245, 301–312.
- Moutray, T.; Evans, J.R.; Lois, N.; Armstrong, D.J.; Peto, T.; Azuara-Blanco, A. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst. Rev. 2018, 1–84.
- Ren, X.; Bu, S.; Zhang, X.; Jiang, Y.; Tan, L.; Zhang, H.; Li, X. Safety and efficacy of intravitreal conbercept injection after vitrectomy for the treatment of proliferative diabetic retinopathy. Eye 2019, 33, 1177–1183.
- Elkjaer, A.S.; Lynge, S.K.; Grauslund, J. Evidence and indications for systemic treatment in diabetic retinopathy: A systematic review. Acta Ophthalmol. 2020, 98, 329–336.
- Aiello, L.P.; DCCT/EDIC Research Group. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014, 37, 17–23.
- Shi, R.; Zhao, L.; Wang, F.; Liu, F.; Chen, Z.; Li, R.; Liu, Y.; Lin, R. Effects of lipid-lowering agents on diabetic retinopathy: A meta-analysis and systematic review. Int. J. Ophthalmol. 2018, 11, 287.
- Knickelbein, J.E.; Abbott, A.B.; Chew, E.Y. Fenofibrate and diabetic retinopathy. Curr. Diabetes Rep. 2016, 16, 1–6.
- Maniadakis, N.; Konstantakopoulou, E. Cost effectiveness of treatments for diabetic retinopathy: A systematic literature review. Pharmacoeconomics 2019, 37, 995–1010.
- Liu, L.; Quang, N.D.; Banu, R.; Kumar, H.; Tham, Y.-C.; Cheng, C.-Y.; Wong, T.Y.; Sabanayagam, C. Hypertension, blood pressure control and diabetic retinopathy in a large population-based study. PLoS ONE 2020, 15, e0229665.
- Hu, H.; Liu, J.; Wang, D.; Qiu, S.; Yuan, Y.; Wang, F.; Wen, L.; Song, Q.; Sun, Z.-L. Efficacy of calcium dobesilate in treating Chinese patients with mild-to-moderate non-proliferative diabetic retinopathy (CALM-DR): Protocol for a single-blind, multicentre, 24-armed cluster-randomised, controlled trial. BMJ Open 2021, 11, e045256.
- Hernández, C.; Simó-Servat, A.; Bogdanov, P.; Simó, R. Diabetic retinopathy: New therapeutic perspectives based on pathogenic mechanisms. J. Endocrinol. Investig. 2017, 40, 925–935.
- Iglicki, M.; Zur, D.; Busch, C.; Okada, M.; Loewenstein, A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: A 24-month cohort study the ‘DR-Pro-DEX Study’. Acta Diabetol. 2018, 55, 541–547.
- Wang, W.; Lo, A.C. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816.
- Wroblewski, J.J.; Hu, A.Y. Topical squalamine 0.2% and intravitreal ranibizumab 0.5 mg as combination therapy for macular edema due to branch and central retinal vein occlusion: An open-label, randomized study. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 914–923.
- Starita, C.; Patel, M.; Katz, B.; Adamis, A.P. Vascular endothelial growth factor and the potential therapeutic use of pegaptanib (Macugen®) in diabetic retinopathy. Diabet. Retin. 2007, 39, 122–148.
- Rodrigues, E.B.; Farah, M.E.; Maia, M.; Penha, F.M.; Regatieri, C.; Melo, G.B.; Pinheiro, M.M.; Zanetti, C.R. Therapeutic monoclonal antibodies in ophthalmology. Prog. Retin. Eye Res. 2009, 28, 117–144.
- Li, H.; Li, B.; Zheng, Y. Exploring the Mechanism of Action Compound-Xueshuantong Capsule in Diabetic Retinopathy Treatment Based on Network Pharmacology. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–12.
- Wei, X.; Balne, P.K.; Meissner, K.E.; Barathi, V.A.; Schmetterer, L.; Agrawal, R. Assessment of flow dynamics in retinal and choroidal microcirculation. Surv. Ophthalmol. 2018, 63, 646–664.
- Laddha, U.D.; Kshirsagar, S.J. Ppar Receptor Modulation And Its Implications In Diabetic Retinopathy: An Overview. J. Crit. Rev. 2020, 7, 3614–3625.
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617.
- Nishijima, K.; Ng, Y.-S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007, 171, 53–67.
- Tolentino, M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv. Ophthalmol. 2011, 56, 95–113.
- Deng, G.; Moran, E.P.; Cheng, R.; Matlock, G.; Zhou, K.; Moran, D.; Chen, D.; Yu, Q.; Ma, J.-X. Therapeutic effects of a novel agonist of peroxisome proliferator-activated receptor alpha for the treatment of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5030–5042.
- Amato, R.; Dal Monte, M.; Lulli, M.; Raffa, V.; Casini, G. Nanoparticle-mediated delivery of neuroprotective substances for the treatment of diabetic retinopathy. Current neuropharmacology 2018, 16, 993–1003.
- Rong, X.; Ji, Y.; Zhu, X.; Yang, J.; Qian, D.; Mo, X.; Lu, Y. Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy in rats. Int. J. Nanomed. 2019, 14, 45.
- Bessone, C.D.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Experimental Eye Research 2020, 200, 108222.
- Shafaie, S.; Hutter, V.; Brown, M.B.; Cook, M.T.; Chau, D.Y. Diffusion through the ex vivo vitreal body–Bovine, porcine, and ovine models are poor surrogates for the human vitreous. Int. J. Pharm. 2018, 550, 207–215.
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Kwon, I.C.; Kim, K. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials 2012, 33, 3485–3493.
More
