2. Immunotherapy
In addition to protein replacement therapy, another branch is immune stimulation against certain diseases. Interleukin 15 (IL-15) cytokine presents a therapeutic anticancer potential, mainly for its immunologic stimulation properties. However, currently used delivery systems with pDNA present low efficiency, and the use of in vitro transcripts could be a better solution. For this, Lei and co-workers, in 2020, verified that through cytokine expression with this mRNA, lymphocyte stimulation was successfully produced and cytotoxicity was triggered in cancer cells. Local or systemic administration of this mRNA induced inhibition of cellular proliferation in several colon cancer models in a safe and efficient way. These results have shown the high therapeutic potential for colorectal cancer immunogenic therapy with this approach
[12][83]. In the same context, Interleukin 2 (IL-2) exerts significant anti-tumor activity. This cytokine is involved in proliferation, differentiation and effector function of T cells and since 1998, it has been approved for the treatment of metastatic melanoma
[13][84]. However, using IL-2 cytokine faces several limitations including the short serum half-life. For this, the use of mRNA expressing IL-2 would prolong the production of the cytokine, thus reducing high and frequent doses. Currently, two nucleoside-modified mRNA LNP encoding for IL-2 are in clinical development for cancer treatment (see review
[14][85]).
Vaccines have been used to provide adequate, specific and short-term immune responses against infectious diseases or cancer. Conventional vaccines consisting of attenuated microorganisms or that contain the majority of virus or bacterial antigens have demonstrated lasting protection against a variety of infectious pathogens, but on rare occasions they can revert to their pathogenic forms
[1][2][1,8]. More and more epidemic outbreaks are caused by viral infections and in all cases, those are characterized by their unpredictability, high morbidity, exponential spread, and substantial social and economic impact
[15][86]. mRNA vaccines, on the other hand, cannot replicate within the body
[1]. Thus, mRNA vaccines have been deeply investigated due to their ability to encode a wide range of antigens, due to the self-adjuvant effects
[2][16][8,23], as well as for their potential large-scale production in a fast, flexible and low-cost manner
[2][16][15][8,23,86]. The development of an mRNA vaccine for specific antigen immunity requires the transfection of antigen-presenting cells, such as dendritic cells
[1][2][17][1,8,87], resulting in the induction of humoral and cytotoxic T-cell response
[15][86] which is represented in
Figure 15. Because of this, the administration is typically performed by intradermal, intramuscular or subcutaneous injection, as dendritic cells are densely found in skeletal muscle and skin tissue
[1][2][4][1,8,29]. In addition to mRNA, DNA was also used for the coding of antigens, but due to the potential for integration into the genome, its use was rather limited
[2][16][8,23].
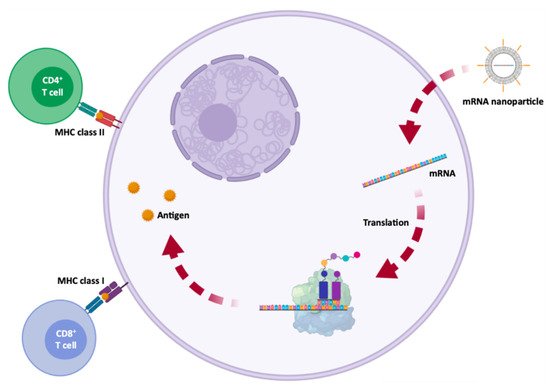
Figure 15. Antigen processing and presentation by dendritic cells, for adaptive immune system activation, following subcutaneous injection of a mRNA vaccine. A synthetic mRNA is internalized by antigen presenting dendritic cells, where the mRNA is translated. Then, the antigen is exposed by class I or II major histocompatibility complex (MHC) molecules and is later recognized by CD8+ or CD4+ T cells, activating chemical and humoral responses.
In contrast to mRNA, antibody-based cancer therapy faces some challenges related to antibody production problems, low stability in long-term storage, aggregation, and the presence of several impurities intrinsic to the production process. In addition, antibodies, especially bispecific antibodies, have a low serum half-life and continuous administration is required to achieve the therapeutic effect
[16][23]. Thus, the use of mRNA for the generation of therapeutic antibodies in patients represents a promising approach, in order to overcome the limitations of direct use of recombinant antibodies
[16][23].
The development of an mRNA vaccine consists of acquiring genetic information of the infectious agent or the sequence of antigens associated with the tumor. Then, the gene is sequenced, synthesized and cloned into a plasmid. The mRNA is transcribed in vitro and the vaccine is administered to the patient
[15][86]. The mRNA vaccine uses the host cell machinery to translate the corresponding antigen mRNA sequence, thus mimicking the infection or a tumor cell, in order to elicit humoral and cytotoxic immune responses
[15][18][86,88]. The use of mRNA to induce adaptive immune responses in cancer began in 1995, with the discovery of protective antitumor immunity, which was obtained by intramuscular injection of mRNA from the carcinoembryonic antigen
[19][89]. There are two main types of mRNA immunotherapy against cancer. The first type of immunotherapy works at the cellular level, in the same way as an mRNA vaccine, however, the mRNA encodes tumor-associated antigens. The second type, on the other hand, involves the modification of T cells, with chimeric antigen receptors (CARs), which is called CAR T cell therapy. Billingsley and collaborators demonstrated the C14-4 LNP induced CAR expression at levels equivalent to electroporation, with a substantially reduced cytotoxicity. When compared to electroporated CAR T cells by the lipid system, C14-4 LNP, in a coculture assay with Nalm-6 acute lymphoblastic leukemia cells, Billingsley and collaborators found that both methods induced a strong cancer-killing activity
[20][90]. These results obtained by Billingsley research group show the progress that has been mad and the promising strategies to deliver mRNA to T cells. Usually, in this class of immunotherapy, the patient’s T cells are transfected with synthetic mRNAs, encoding CARs, bind to specific tumor antigens, subsequently eliminating the tumor cells
[1].
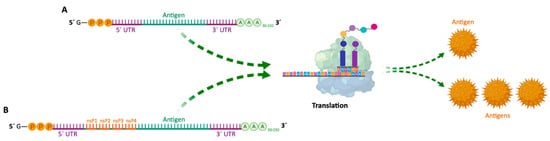
Since then, mRNA vaccines have been classified into two subtypes: (i) non-amplifying mRNA-based vaccines (also known as mRNA conventional vaccines), that encode the antigen of interest and contain the 5′ and 3′ UTRs
[18][88]; and (ii) self-amplifying mRNA (SAM or saRNA) vaccines
[2][21][15][22][8,13,86,91] that not only encode the antigen, but also the viral replication mechanism, allowing an increase in the amount of intracellular mRNA, consequently leading to a more abundant protein expression
[18][88] (
Table 23 and
Figure 26). Both types of mRNA vaccines use the translation mechanism of host cells to produce target antigens, in order to induce specific adaptive immune responses
[2][8]. In 2020, He and co-workers developed cationic nanolipoprotein particles (NLPs) to enhance the delivery of large self-amplifying mRNAs (replicons) in vivo. These cationic lipids successfully encapsulated RNA encoding luciferase, protected it from RNase degradation and promoted replicon expression in vivo
[23][92].
Figure 26. (A)—Schematic structure of conventional non-amplifying mRNA vaccine. (B)—Schematic structure of self-amplifying mRNA vaccine (replicon). UTR—Untranslated region; nsP—Non-structural proteins; A—Adenine; G—Guanine; P—Phosphate group.
Table 23. Advantages and disadvantages of non-amplifying mRNA Vaccines and SAM Vaccines.
| mRNA Vaccines |
Structure |
Advantages |
Disadvantages |
References |
| Non-amplifying mRNA Vaccines |
Basic structure of the mRNA, with a coding region for the desired antigens. |
- Relatively small mRNA size (~2–3 kb).
- Absence of additional proteins, minimizing unwanted immune interactions.
- Relatively easy to produce and amplify.
- Simplified sequence engineering.
- Direct antigen expression. |
- Potential toxicity from modified nucleotides.
- Short duration of expression.
- Need for high RNA doses.
- Low antigen quantity. |
[ |
][21][8,13]. Although therapies based on dendritic cells still represent the majority of clinical trials of mRNA vaccines, vaccination with the use of this biomolecule through non-viral vectors, as well as gene editing, is increasingly being investigated in search of new therapies against diverse diseases
[1][2][1,8] (
Table 34).
Table 34. Clinical trials for RNA-Based Protein Therapy (Protein replacement, cell reprogramming, immunotherapy) and gene editing.
| Name |
Therapetic Modality |
Protein Target |
Administration Method |
Delivery Vehicle |
Disease |
Sponsor Institution |
ClinicalTrials.gov Identifer |
Phase |
Therapeutic Approach |
References |
| MRT5005 |
mRNA | 2 |
CFTR][ |
Inhalation15] |
| LNPs |
Cystic fibrosis |
Translate Bio |
NCT03375047 |
SAM Vaccines |
Encode a manipulated RNA virus genome (replicon). It generally contains two different protein coding regions, one encoding nonstructural proteins involved in mRNA capping and replication, and the other in antigen expression. |
- High yield of target antigen.
- Enhanced and prolonged antigen expression.
- Lower effective RNA doses (more safe).
- Intrinsic adjuvant effect.
- Potential apoptosis of vaccine-carrying cells due to vaccine self-amplification (enhanced cross-presentation).
- Option for single-vector delivery of multiple or complex antigens. |
- RNA replicons are not able to tolerate many of the synthetic nucleotide modifications and sequence alterations.
- Inclusion of unrelated proteins, which may increase unwanted immunogenicity.
- Large replicon size (~10 kb), decreasing cell internalization efficiency.
- Interaction between nsPs and host factors yet to be addressed.
- Longer RNA length (more difficult production).
- Potential elevated inflammation. |
[2][21][4][15][24] |
Advantages and disadvantages of non-amplifying mRNA Vaccines and SAM Vaccines.
The greatest barrier to the usefulness of these vaccines is the need for intracellular delivery
[2][21][8,13]. However, as already mentioned, through chemical modifications, encapsulation by nanoparticle formulations and through sequence engineering, it is possible to promote an improved targeting, delivery and entrance into the cell, in addition to greater efficiency in translation and enhanced half-life of synthetic mRNA vaccines
[2][8]. Chronologically, mRNA vaccines in dendritic cells for adaptive immunotherapy against cancer and protein replacement therapies were the first therapeutic applications with these biomolecules to enter clinical trials
[2
| Protein Replacement |
[ | 25 | ] |
| AZD8601 |
mRNA |
VEGF-A |
Intracardiac injection |
Naked mRNA |
Heart failure |
AstraZeneca |
NCT03370887 |
II |
Cell reprogramming |
[26] |
| CV7201 |
mRNA |
Rabies virus glycoprotein |
I.D or I.M |
RNActive, protamine |
Rabies |
CureVac |
NCT02241135 |
I |
Immunotherapy |
[27] |
| CV7202 |
mRNA |
Rabies virus glycoprotein |
I.M |
LNPs |
Rabies |
NCT03713086 |
I |
Immunotherapy |
[21] |
| CV9201 |
mRNA |
TAAs |
I.D |
RNActive, protamine |
NSCLC |
NCT00923312 |
I/II |
Immunotherapy |
[28] |
| CV9202 |
mRNA |
TAAs |
I.D |
RNActive, protamine |
NSCLC |
NCT03164772 |
I/II |
Immunotherapy |
[2] |
| CV9104 |
mRNA |
TAAs |
I.D |
RNActive, protamine |
Prostate carcinoma |
NCT02140138 |
II |
Immunotherapy |
| HARE-40 |
mRNA |
HPV antigen CD40 |
I.D |
Naked RNA |
HPV-driven squamous cell
carcinoma |
BioNTech |
NCT03418480 |
I/II |
Immunotherapy |
| Lipo-MERIT |
mRNA |
TAAs: NYESO-1, MAGE-A3, tyrosinase, and TPTE |
I.V |
Lipo-MERIT,
DOTMA(DOTAP)/
DOPE lipoplex |
Advanced melanoma |
NCT02410733 |
I |
Immunotherapy |
| IVAC |
mRNA |
3 TAAs selected from a warehouse and p53 RNA; Neo-Ag based on NGS screening |
I.V |
Lipo-MERIT,
DOTMA(DOTAP)/DOPE lipoplex |
TNBC |
BioNTech |
NCT02316457 |
I |
Immunotherapy |
[2] |
| RBL001/RBL002 |
mRNA |
TAAs |
Ultrasound guided
I.N |
Naked mRNA |
Melanoma |
NCT01684241 |
I |
Immunotherapy |
| - |
- |
- |
Gennova |
- |
Pre-Clinical |
IVAC MUTANOME |
mRNA |
Neo-Ag |
Ultrasound guided |
| mRNA |
| I.N |
Naked mRNA |
Melanoma |
NCT02035956 |
I |
Immunotherapy |
| mRNA |
- |
- |
- |
Selcuk University |
- |
Pre-Clinical |
RO7198457 |
mRNA |
Neo-Ag |
I.V |
Naked mRNA |
Melanoma; NSCLC; Bladder cancer |
NCT03289962 |
I |
| LNP-mRNA |
mRNA | Immunotherapy |
| - |
- |
LNP |
Translate Bio/Sanofi Pasteur |
- |
Pre-Clinical |
mRNA-1325 |
mRNA |
Zika virus antigen |
I.D |
LNPs |
Zika virus |
Moderna |
| LNP-mRNA |
mRNA |
- | NCT03014089 |
I |
Immunotherapy |
| - |
LNP |
CanSino Biologics/Precision NanoSystems |
- |
mRNA-1653 |
mRNA |
hMPV and hPIV type 3 vaccine |
I.D |
LNPs |
hMPV and
hPIV infection |
NCT03392389 |
I |
Immunotherapy |
| Pre-Clinical |
| LNP-encapsulated mRNA cocktail encoding VLP |
mRNA |
- |
- |
LNP |
Fudan University/Shanghai JiaoTong University/RNACure Biopharma |
- |
Pre-Clinical |
VAL-506440 |
| LNP-encapsulated mRNA encoding RBDmRNA |
H10N8 antigen |
I.D |
LNPs |
Influenza |
Moderna |
NCT03076385 |
I |
Immunotherapy |
[29] |
| mRNA |
RBD |
- |
LNP |
Fudan University/Shanghai JiaoTong University/RNACure Biopharma |
- |
Pre-Clinical |
VAL-339851 |
mRNA |
H7 influenza antigen |
I.D |
LNPs |
Influenza |
NCT03345043 |
I |
Immunotherapy |
| Replicating Defective SARS-CoV-2 derived RNAs |
mRNA |
- |
- |
- |
Centro Nacional Biotecnología (CNB-CSIC), Spain |
- |
Pre-Clinical |
mRNA-1647/1443 |
mRNA |
CMV glycoprotein H pentamer complex |
I.D |
LNPs |
CMV infection |
| LNP-encapsulated mRNA |
mRNA |
- |
- |
LNP |
University of Tokyo/Daiichi-SankyoNCT03382405 |
I |
Immunotherapy |
[2] |
| - |
Pre-Clinical |
mRNA-2416 |
mRNA |
Human OX40L |
I.D |
LNPs |
Solid tumor malignancies or |
| Liposome-encapsulated mRNA |
mRNA |
| lymphoma |
-NCT03323398 |
I |
Immunotherapy |
| - |
LNP |
BIOCAD |
- |
Pre-Clinical |
mRNA-4157 |
mRNA |
Neo-Ag |
Intratumoral |
LNPs |
Solid tumor |
| Several mRNA candidates |
mRNA |
- | NCT03313778 |
I |
Immunotherapy |
| - |
- |
RNAimmune, Inc. |
- |
Pre-Clinical |
mRNA-4650 |
mRNA |
Neo-Ag |
I.M |
Naked mRNA |
Melanoma;
Colon cancer;
GI cancer; |
| mRNA |
mRNA |
|
-Genitourinary cancer; |
-
HCC |
-NCT03480152 |
I/II |
Immunotherapy |
FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo |
- |
Pre-Clinical[30][31] |
| mRNA-1388 |
mRNA |
VAL-181388 |
I.M |
LNPs |
CHIKV |
NCT03325075 |
I |
Immunotherapy |
| mRNA |
mRNA |
- |
- |
-[32] |
| China CDC/Tongji University/Stermina |
- |
Pre-Clinical |
mRNA-2752 |
mRNA |
OX40L, IL-23, and IL-36γ |
Intratumoral |
LNPs |
Solid tumor or lymphoma |
Moderna/AstraZeneca |
NCT03739931 |
I |
Immunotherapy |
[21] |
| iHIVARNA-01 |
mRNA |
Trimix (CD40L, CD70 and caTLR4 RNA—mRNA-transfected) |
I.N |
Naked mRNA |
HIV infection |
Hospital Clínic de
Barcelona |
NCT02413645 |
I |
Immunotherapy |
[33] |
| mRNA |
I.N |
Naked mRNA |
HIV infection |
Erasmus Medical Center |
| mRNA in an intranasal delivery system |
mRNA |
- |
Intranasal |
- |
eTheRNA |
- |
Pre-Clinical |
| mRNA |
mRNA |
- |
- |
- |
Greenlight Biosciences |
- |
Pre-Clinical |
NCT02888756 |
II |
Immunotherapy |
[34] |
| mRNA |
mRNA |
- |
- |
- |
IDIBAPS-Hospital Clinic, Spain |
- |
Pre-Clinical |
- |
mRNA |
CT7, MAGE-A3, and WT1 mRNA-electroporated LCs |
I.D |
DC-loaded mRNA |
Malignant melanoma |
Memorial Sloan
Kettering Cancer
Center |
NCT01995708 |
I |
Immunotherapy |
| mRNA |
mRNA | [ | 2 | ] |
| - |
- |
- |
Providence Therapeutics |
- |
Pre-Clinical |
- |
mRNA |
HIV-1 Gag- and Nef-transfected DCs |
I.D |
DC-loaded mRNA |
HIV infection |
Massachusetts
General Hospital |
NCT00833781 |
I/II |
Immunotherapy |
[ |
| mRNA |
mRNA |
- |
- | 35 |
- |
Cell Tech Pharmed |
-] |
| Pre-Clinical |
- |
mRNA |
Neo-Ag |
S.C |
Naked mRNA |
Solid tumor malignancies or
lymphoma |
Changhai Hospital
Stemirna
Therapeutics |
NCT03468244 |
N.A |
| mRNA |
mRNA |
- | Immunotherapy |
[ | 2 | ] |
| - |
- |
ReNAP Co. |
- |
mRNA |
TAA for melanoma (Melan-A, MAGE-A1, MAGE-A3,
survivin, GP100, and tyrosinase) |
I.D |
Naked mRNA |
Melanoma |
University Hospital
Tuebingen |
NCT00204516 |
I/II |
Immunotherapy |
[32] |
| - |
mRNA |
TAA-transfected DC |
I.D or I.N |
DC-loaded mRNA |
Malignant melanoma |
Oslo University
Hospital |
NCT01278940 |
I/II |
Immunotherapy |
[36] |
| - |
mRNA |
I.D |
DC-loaded mRNA |
Prostate cancer |
NCT01278914 |
I/II |
Immunotherapy |
[2] |
| AVX601 |
Replicon |
Alphavirus replicon vaccine expressing CMV
genes |
I.M or S.C |
- |
CMV |
AlphaVax |
NCT00439803 |
I |
Immunotherapy |
[2] |
| AVX502 |
Replicon |
Alphavirus replicon vaccine expressing an influenza
HA protein |
I.M or S.C |
- |
Influenza |
NCT00440362;
NCT00706732 |
I/II |
Immunotherapy |
| AVX101 |
Replicon |
Alphavirus replicon, HIV-1 subtype C Gag vaccine |
I.M or S.C |
- |
HIV infections |
NCT00097838; NCT00063778 |
I |
Immunotherapy |
[37] |
| AVX701 |
Replicon |
Alphavirus replicon encoding the protein |
I.M or S.C |
- |
Colon cancer;
CRC;
Breast cancer;
Lung cancer;
Pancreatic cancer |
NCT01890213;
NCT00529984 |
I/II |
Immunotherapy |
[2] |
| NY-ESO-1 |
CRISPR-Cas9 |
PD-1 and TCR |
Ex vivo |
Autologous T cells |
Multiple myeloma; Synovial sarcoma;
Melanoma |
University of Pennsylvania |
NCT03399448 |
I |
Gene Editing |
[2] |
| CRISPR/TALEN-HPV E6/E7 |
CRISPR/Cas9, TALEN |
E6 and E7 |
N.A |
Plasmid DNA in gel |
Cervical intraepithelial neoplasia |
First Affiliated Hospital, Sun Yat-Sen University |
NCT03057912 |
I |
Gene Editing |
[38][39] |
[22][56][88,91,123]. After transfection of either muscle cells or dendritic cells, the expressed S glycoproteins are presented by the major histocompatibility complex (MHC) class I and II
[46][113]. This process stimulates humoral immunity and leads to the production of neutralizing antibodies against the S glycoprotein by B lymphocytes, preventing viral binding and entry into cells
[55][122] represented in
Figure 48. It also induces the generation of specific cytotoxic T cells (CD8
+) which can eradicate SARS-CoV-2-infected cells
[57][58][124,125].
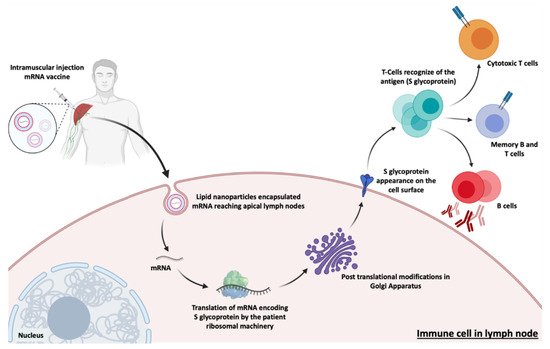
Figure 48. Actuation mechanism of the main mRNA vaccines against SARS-CoV-2. This process begins with the injection, in the patient’s deltoid muscle, of the mRNA usually encapsulated in lipid nanoparticles (LNPs). LNPs loaded with the mRNA encoding the SARS-CoV-2 spike glycoprotein (S), can reach the apical lymph nodes where they transfect dendritic cells. After entry and release of the cell endosome, the mRNA sequence is expressed and post-translational modifications occur. Subsequently, the S glycoprotein is transported and presented in the cell membrane of immune cells (antigen presenting cells). The S glycoproteins incites a specific cytotoxic and humoral immune response, leading to the production of antibodies against SARS-CoV-2, with the aim of achieving immunization against COVID-19.
To date, the Pfizer/BioNTech (Comirnaty) vaccine presents 95% of efficiency, while the Moderna (Spikevax) vaccine has 94.5%, Gamaleya product has 92% and the AstraZeneca vaccine, 70%
[59][60][126,127]. The first two (Pfizer/BioNTech and Moderna) are RNA vaccines that express COVID-19 spike glycoprotein, while the Gamaleya and AstraZeneca vaccines express spike protein from adenovirus vector platforms
[61][62][128,129].
Both Moderna and Pfizer/BioNTech vaccines are made of a nucleoside-modified (N1 methyl pseudouridine) mRNA formulated in LNPs. These LNPs contain an ionizable lipid, neutral/auxiliary lipid ((phospholipid distearoylphosphatidylcholine) (DSPC)) at physiological pH and cholesterol, which allows to stabilize LNP and increase the efficiency of mRNA delivery, and finally a polyethylene glycol (PEG), which aims to improve colloidal stability, reducing opsonization by plasma proteins. However, these differ in the use of the ionizable lipid, as the Pfizer/BioNTech vaccine used the ionizable lipid ALC-0315 and Moderna vaccine used another ionizable lipid, the SM-102 (
Table 45). Although both vaccines use ionizable lipids, both are tertiary amines that are protonated at a low pH, thus allowing for mRNA interaction and protection
[63][64][130,131] (
Table 45). These specific LNPs are therefore essential for a safe and efficient immune response. The mRNA encodes the membrane-anchored, full-length SARS-CoV-19 spike protein and contains mutations for the prefusion conformation, which stabilize the Spike protein.
Table 45. LNP carriers of the COVID-19 mRNA vaccines (lipidic constituents) [63][64].
| Lipid Name |
Role |
Abbreviation |
Molar Lipid Ratios (%) (Ionizable Cationic Lipid:Neutral Lipid:Cholesterol:PEG-ylated Lipid) |
| BNT162b2 vaccine |
| 4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate |
ionizable cationic lipid |
ALC-0315 |
46.3:9.4:42.7:1.6 |
,141,142,143]. These improved properties conferred by the incorporation of Ψ make mRNA a promising tool for both gene replacement and vaccination. The innate immune system cells are activated by RNA, since it stimulates Toll-like receptors (TLRs), namely TLR3, TLR7, and TLR8. However, when some modified nucleosides, like, Ψ, 5-methylcytidine (m5C), N6-methyladenosine (m6A), 5-methyluridine (m5U), or 2-thiouridine (s2U) were included into the transcript, most of TLRs were no longer activated, therefore controlling immune activation in vitro and in vivo
[74][19]. These characteristics and the readiness of producing such RNAs by in vitro transcription make Ψ-containing mRNA an important tool for the expression of any protein
[78][79][80][81][144,145,146,147]. Furthermore, codon optimization strategies have been investigated to improve the cost efficiency of recombinant protein production, once most amino acids are encoded by different codons. This is primarily based on the substitution of multiple rare codons by others more frequent, that encode the same amino acid, thus resulting in increased rate and efficiency of protein translation
[82][148]. Another successful modification is the addition of a poly(A) to IVT produced mRNA, which can be directly added during the transcription process (if the DNA template encodes the poly(T) sequence) or can be added post-transcriptionally by enzymatic reactions. Poly(A) tail length influences stability and translation efficiency. Even with a relatively long poly(A) tail that seems to be appropriate, the optimal length can vary depending on the target cell
[5][83][43,149]. The Kozak sequence plays a major role in the initiation of the translation process and is located at the 5′ UTR. This sequence, defined as “RCCAUGG”, where “R” stands for a purine (A or G), helps drive high levels of translation from the correct start codon, therefore being considered the election sequence for translation initiation in eukaryotes
[5][81][43,147]. The Pfizer/BioNTech vaccine included a poly(A) chain in their mRNA sequence, as well as an optimized Kozak sequence
[81][147].
Conventional vaccine approaches, such as the use of attenuated and inactivated viruses, successfully provide durable protection against infectious diseases, but they are not able to meet the need for rapid and large-scale development. As already mentioned, although genetic immunization, such as DNA vaccines, has shown to be promising, pDNA delivery raises safety concerns due to the possibility of insertional mutagenesis. Thus, in order to try to obtain a vaccine quickly, safely and effectively, the development of an mRNA vaccine seems to be a reliable approach. This is a safer alternative, as it does not require entry into the nucleus for translation to occur, leading to an improvement in transfection and expression efficiency compared to DNA vaccines. It also presents comparatively lower production costs and capacity for rapid development, because with a simple change of the mRNA sequence, it will lead to the expression of a different protein, which is beneficial given the frequent viral mutations
[22][45][91,112]. There are currently eight mRNA-based vaccines in clinical development and 22 in pre-clinical studies (
Table 56)
[84][150].
Table 56. mRNA vaccines and new candidates for COVID-19 [84].
| Name |
Therapetic Modality |
Protein Target |
Administration Method |
Delivery Vehicle |
Developer |
ClinicalTrials.gov Identifer, EU Clinical Trials Register or Chinese Clinical Trial Register |
Phase |
| mRNA-1273 |
mRNA |
Spike Glycoprotein |
Intramuscular |
LNP |
Moderna/NIAID |
EUCTR2021-002327-38-NL |
IV |
| BNT162b2 |
mRNA |
RBD/Spike Glycoprotein |
Intramuscular |
LNP |
Pfizer/BioNTech + Fosun Pharma |
NCT04760132 |
IV |
1,2-Distearoyl-sn-glycero-3-phosphocholine |
helper lipid |
DSPC |
| cholesterol |
helper lipid |
Chol |
| 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide |
PEG-lipid |
ALC-0159 |
| mRNA-1273 vaccine |
| heptadecan-9-yl 8-((2-hydroxyethyl)[6-oxo-6-(undecyloxy)hexyl]amino)octanoate |
ionizable cationic lipid |
SM-102 |
| CTX001 |
| CRISPR-Cas9 |
| BCL11A |
| Ex vivo |
| Modified CD34 |
| + |
| hHSPCs |
| ß-thalassemia |
| Vertex Pharmaceuticals Incorporated |
| NCT03655678 |
| I/II |
| Gene Editing |
| [ |
| 2 |
| ] |
| - |
CRISPR-Cas9 |
PD-1 and TCR |
Ex vivo |
CAR-T cells |
Mesothelin positive multiple solid tumors |
Chinese PLA General Hospital |
NCT03545815 |
I |
Gene Editing |
| - |
CRISPR-Cas9 |
CD19 and CD20 |
Ex vivo |
Dual specificity CAR-T cells |
ß cell leukemia and lymphoma |
NCT03398967 |
I/II |
Gene Editing |
| UCART019 |
CRISPR-Cas9 |
CD19 |
Ex vivo |
CAR-T cells |
ß cell leukemia and lymphoma |
NCT03166878 |
I/II |
Gene Editing |
[40] |
| - |
CRISPR-Cas9 |
PD-1 |
Ex vivo |
Cytotoxic T lymphocytes |
EBV-associated
malignancies |
Yang Yang |
NCT03044743 |
I/II |
Gene Editing |
[41][42] |
| SB-728mR-HSPC |
ZFN mRNA |
CCR5 |
Ex vivo (mRNA) |
CD34+ hHSPCs |
HIV |
City of Hope Medical Center |
NCT02500849 |
I |
Gene Editing |
[43] |
| SB-728mR-T |
ZFN mRNA |
CCR5 |
Ex vivo (mRNA) |
T cells |
HIV |
Sangamo Therapeutics |
NCT02225665 |
I/II |
Gene Editing |
[44] |
Clinical trials for RNA-Based Protein Therapy (Protein replacement, cell reprogramming, immunotherapy) and gene editing.
The most recent case of immunotherapy associated to mRNA vaccination, in clinical trials, was registered in 2020 and concerns the virus named “Serious Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)”, the former being known as Coronavirus (COVID-19 or 2019-nCoV)
[45][46][112,113]. SARS-CoV-2 causes an infection in the alveolar epithelial cells of the human respiratory tract
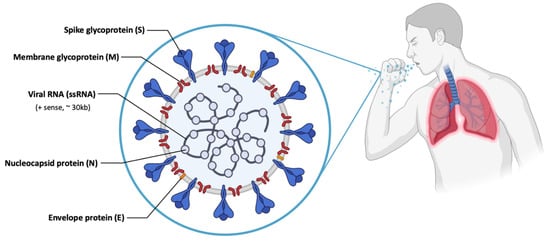
[47][48][114,115]. SARS-CoV-2 has a large genetic structure. The genome is surrounded by helical nucleocapsid proteins (N) and an outer envelope composed of matrix or membrane glycoproteins (M), envelope proteins (E) and spike glycoproteins (S) (
Figure 37), which improve binding to cells, transport and interfere with the immune response of the host
[22][49][50][91,116,117]. In addition, the virus has several non-structural proteins (NsPs) that are vital for its life cycle and pathogenic character
[22][91].
Figure 37. Scheme of the structure of SARS-CoV-2, with different viral proteins indicated.
The S glycoprotein is part of the outer layer of the virus and is essential for its entry into cells
[51][118]. This protein consists of a receptor binding domain (RBD), that is responsible for specific binding to the angiotensin-converting enzyme 2 (ACE2) receptor, thus allowing the entry of SARS-CoV-2
[47][114] in the epithelium cells of human lung
[52][119]. In addition, there are studies that indicate that SARS-CoV-2, as well as SARS-CoV, can enter the cell through clathrin-mediated endocytosis
[48][115]. Of all structural proteins, it was found that S glycoprotein induces neutralizing antibodies and it was the main target antigen for the development of the vaccines
[50][53][117,120].
Given the high transmission of SARS-CoV-2, the World Health Organization (WHO) emphasized the demand for a rapid response to this situation, endeavoring the immediate development of safe and effective prophylactic therapies
[45][54][112,121]. Due to the great technological advances in sequencing techniques, it was possible to obtain colossal knowledge about SARS-CoV-2 in a very short time, something unprecedented in the history of medicine
[46][51][113,118].
Vaccines decrease the viral spread and transmission from person to person
[18][22][88,91], and the development of the SARS-CoV-2 mRNA vaccine was impressively fast
[55][122]. The classical development of vaccines requires an average of about 5 to 10 years, but given the need and technological advances, the development time of the vaccine against SARS-CoV-2 was substantially shorter
[18][46][88,113].
The approved mRNA vaccines to combat SARS-CoV-2, the vaccines developed by Moderna/NIAID and BioNTech/Fosun Pharma/Pfizer, aim at the expression of the S glycoprotein or RBD subunit
[18]
| 1,2-distearoyl-sn-glycero-3-phosphocholine |
| helper lipid |
| DSPC |
| cholesterol |
helper lipid |
Chol |
| 1,2-Dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 |
PEG-lipid |
PEG2000-DMG |
LNP carriers of the COVID-19 mRNA vaccines (lipidic constituents) [130,131].The LNPs prevent RNA degradation and enable its delivery into host cells after intramuscular injection. Once inside the host cells, mRNA is translated into SARS-CoV-2 spike protein. The expression of this spike antigen induces neutralizing antibodies, as well as cellular immune responses against it, which can confer protection against COVID-19
[65][132]. The Pfizer/BioNTech vaccine has been recommended to people older than 12 years old, with a dose of 30 μg (0.3 mL) at a cost of $19.50 in the US. Recently, FDA issued emergency use authorization in individuals 5 years of age and older
[66][133]. It consists of a two-dose administration with 21 days between each administration, providing immunogenicity for at least 119 days after the first vaccination and is 95% effective at preventing the SARS-CoV-2 infection. However, the Moderna Vaccine (mRNA-1273) has been recommended to people of or above 18 years of age, with a dose of 50 μg (0.5 mL) at a cost ranging from $32 and $37, in the US
[67][134]. Similarly, it also consists of two shots administered 28 days apart providing immunogenicity for at least 119 days after the first vaccination and is, as referred above, 94.5% effective in the prevention of SARS-CoV-2 infection. It should be noted that age-dependent administration is region-specific, and can vary in different countries
[68][69][135,136]. Given this, it is safe to say that both vaccines are beneficial in providing immunity against SARS-CoV-2 infection, nevertheless, some allergic responses have been reported. These COVID-19 vaccines can cause mild adverse effects after the first or second dose, including pain, redness, swelling or itching (at the site of vaccine injection), fever, fatigue, headache, muscle pain, nausea, and rarely cause anaphylactic shock. The Pfizer/BioNTech vaccine reports a lower percentage of these adverse effects comparatively with the Moderna vaccine. However, the Moderna vaccine is easier to transport and store (storage between −25 °C and −15 °C) because it is less sensitive when compared to the Pfizer vaccine (stored between −80 °C and −60 °C)
[60][70][71][127,137,138].
CVnCoV (CureVac) consists of LNP-encapsulated non-chemically modified mRNA with naturally occurring nucleotides encoding for a full-length S protein that includes two proline mutations (S-2P), which was previously showed to stabilize the conformation of the S proteins for SARS-CoV. The mRNA was codon-optimized in order to provide a higher expression level of S protein and a moderate activation of the immune system
[72][139]. The CureVac vaccine can be distinguished from the previous two candidates by exclusively consisting of non-chemically modified nucleotides and can be applied at comparatively lower doses (12 μg). CureVac company announced preliminary data on 16 June (from a 40,000—person trial), that its two-dose vaccine was only 47% effective at preventing COVID-19, which is half of the efficiency of its previous two rivals. It was expected that this third vaccine candidate would be cheaper and last longer in refrigerated storage than the earlier mRNA vaccines made by Pfizer/BioNTech and Moderna. However, it is suspected that CureVac’s decision not to exchange the biochemical structure of its mRNA, as Pfizer/BioNTech and Moderna did, might be the reason for its poor performance
[72][73][139,140].
It should be noted that clinical application of mRNA as a therapeutic agent has some limitations due to its instability and the capacity to activate the immune system. Therefore, modifying the in vitro transcribed mRNA structure alongside with the design of suitable nanoparticles is of great importance
[5][43]. This fact comes to be noticed because Pfizer/BioNTech and Moderna vaccines call upon modified RNA, by replacing uridine itself for another nucleotide called pseudouridine (Ψ), which is similar to uridine but contains a natural modification. This modification in exogenous mRNA is thought to decrease inflammatory reactions, while improving translational efficiency and stability. In contrast to Pfizer/BioNTech and Moderna vaccines, CureVac uses normal uridine instead of Ψ, which could be a reason for its poor success once higher doses reflected more severe adverse effects
[74][75][76][77][19
| CVnCoV Vaccine |
| mRNA |
Spike Glycoprotein |
Intramuscular |
LNP |
CureVac AG |
NCT04674189 |
III |
| ARCT-021 |
mRNA |
Spike Glycoprotein |
Intramuscular |
LNP |
Arcturus Therapeutics |
NCT04668339 |
II |
| LNP-nCoVsaRNA |
mRNA |
Spike Glycoprotein |
Intramuscular |
LNP |
Imperial College London |
ISRCTN17072692 |
I |
| SARS-CoV-2 mRNA vaccine (ARCoV) |
mRNA |
RBD |
Intramuscular |
LNP |
AMS/Walvax Biotechnology and Suzhou Abogen Biosciences |
NCT04847102 |
III |
| ChulaCov19 mRNA vaccine |
mRNA |
Spike Glycoprotein |
Intramuscular |
LNP |
Chulalongkorn University |
NCT04566276 |
I |
| PTX-COVID19-B, mRNA vaccine |
mRNA |
Spike Glycoprotein |
Intramuscular |
LNP |
Providence therapeutics |
NCT04765436 |
I |
| saRNA formulated in a NLC |
mRNA |
- |
- |
NLC |
Infectious Disease Research Institute/Amyris, Inc. |
- |
Pre-Clinical |
| LNP-encapsulated mRNA encoding S |
mRNA |
Spike Glycoprotein |
- |
LNP |
Max-Planck-Institute of Colloids and Interfaces |
- |
Pre-Clinical |
| Self-amplifying RNA |
mRNA |
| - |
| Pre-Clinical |
| D614G variant LNP-encapsulated mRNA |
| mRNA |
- |
- |
LNP |
Globe Biotech Ltd. |
- |
Pre-Clinical |
| Encapsulated mRNA |
mRNA |
- |
- |
- |
CEA |
- |
Pre-Clinical |
mRNA vaccines and new candidates for COVID-19 [150].3. Gene Editing
As previously mentioned, mRNA therapies may also function in gene editing, which can be achieved by encoding nucleases from mRNA for cellular reprogramming
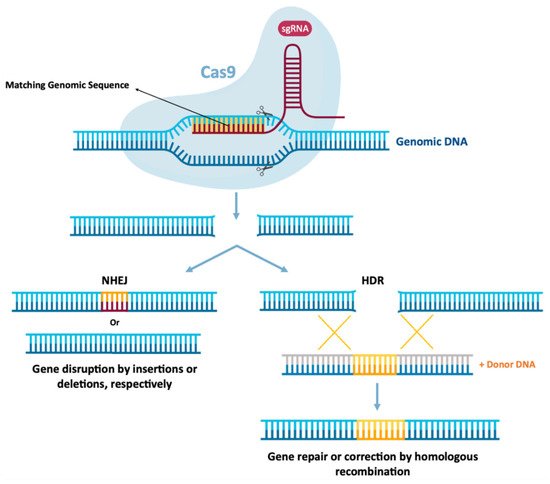
[1][21][1,13]. Gene editing involves the precision of “cutting” and “pasting” genomic DNA in specific locations, expecting the establishment of a potentially permanent cure for genetic diseases to be a promising therapeutic area for the application of mRNA technology
[1][2][1,8]. In this therapeutic area, mRNA function is to express programmable nucleases, including zinc finger nucleases (ZFNs), transcription activator effector nucleases (TALENs)
[2][21][8,13] or CRISPR-Cas9
[1][2][1,8]. These genetic engineering tools allow the replacement or modification of gene expression, through the introduction or local deletion of specific modifications in the genome of target cells
[2][8]. This allows the correction of a target gene, by deleting disease-causing mutations or by inserting protective mutations by joining the non-homologous end (NHEJ)
[85][86][151,152] or even performing a repair or insertion directed to homology (HDR)
[85][86][87][151,152,153]. This is schemed in
Figure 59. ZFNs and TALENs facilitate the recognition of a sequence by protein–DNA interactions
[2][21][8,13], however, the complex engineering necessary to create specific domains in proteins directed to DNA recognition and binding greatly restricts its wide application. However, the CRISPR-Cas9 system in
Figure 59, is currently the most widely used and characterized gene editing technology
[1][2][1,8].
Figure 59. Schematic of CRISPR-Cas9-mediated genome editing. A CRISPR-Cas9 endonuclease is directed to a DNA sequence by means of a single guide RNA sequence (sgRNA), resulting in double strand cleavage. Subsequently they are repaired by non-homologous final union (NHEJ) or homology-directed repair (HDR). NHEJ repair provides errors, often leads to insertion or deletion mutations, which can lead to genome instability. Alternatively, in the presence of an exogenous donor DNA model, it can be repaired through error-free HDR, projecting precise DNA changes.
In 2020, Jennifer Doudna and Emmanuelle Charpentier won the 2020 Nobel Chemistry Prize for their discovery of a novel and innovative gene-editing technique. CRISPR-Cas9 gene-editing tools allow precise editing of the genome and have countless applications, which scientists aim to use to alter human genes to eliminate diseases and eradicate pathogens. CRISPR-Cas9-mediated gene editing requires only two components: Cas9, a nuclease responsible for DNA cleavage and a short single-stranded RNA guide (sgRNA), which directs DNA cleavage by the nuclease, precisely. Typically, these two components are delivered to cells using a pDNA containing the Cas9 protein and sgRNA genes
[1][87][1,153]. For more information on the mechanism of action of the CRISPR-Cas9 system, the following literature can be analyzed
[85][86][87][151,152,153]. The use of CRISPR-Cas9 technology had only been used to edit the genomes of embryos, zygotes, and cultured cells
[88][89][154,155], however, this technology has been increasingly used in vivo.
Due to the transient nature of mRNA, the use of this biomolecule can be advantageous in relation to the use of pDNA
[1], limiting the presence of nucleases inside cells
[21][13]. In this way, there is a reduction in possible non-specific cleavages which decreases the immune response to the Cas9 protein. In addition to these advantages, it appears that the intracellular presence of the Cas9 protein has been more persistent after mRNA expression compared to the administration of the Cas9/sgRNA ribonucleoprotein complex (Cas9-RNP)
[1][2][1,8]. As such, co-delivery of mRNA, which encodes Cas9, and sgRNA is an attractive alternative
[1]. Cas9 can be administered as mRNA, plasmid DNA or even as a protein. However, for plasmid DNA Cas9 to be functional it must overcome cell and nuclear membrane barriers. Thus, an alternative approach could be the use of Cas9 mRNA. This approach becomes preferable as mRNA only needs to cross the cell membrane to be functional. Liang and collaborators, using the GeneArt commercial system (Thermo) and electroporation, found that in the study of eleven cell lines, the delivery of Cas9 mRNA/gRNA or Cas9 RNPs was superior to the plasmid delivery in all cell lines tested. They also noticed that although the similar cleavage kinetics between Cas9 delivered as plasmid DNA, mRNA and protein to HEK293 cells, in cells transfected with plasmid DNA, the Cas9 protein accumulated over time, while the relatively low expression of Cas9 in mRNA-transfected cells seemed relatively stable for approximately 48 h. However, due to the fast turnover of Cas9 RNP and mRNA compared to the long persistence of Cas9 expressed from plasmids, this could reduce the opportunity for off-target binding and cleavage. When studying this among the six potential off-target sites, it was observed that the use of mRNA and Cas9 RNP had much smaller off-target effects than the use of Cas9 from plasmid DNA
[90][156]. In addition to the use of mRNA in gene editing for the treatment of acquired diseases, Mohsin and colleagues demonstrated by in vitro experiments that Cas9 mRNA/sgRNAs can reduce the sporulation percentage of
E. tenella oocysts, as well as their survival rate. These data show that the use of a highly specific sgRNA molecule, when combined with Cas9 mRNA, may be a potentially powerful agent in the development of new therapeutic drugs against parasitic diseases
[91][157]. It should be reinforced that for long-term gene therapy purposes, mRNAs are not sufficiently stable; nevertheless, even transient articulation and ability will make hereditary change perpetual for the activity of Cas9 nuclease. This is the reason why Cas9 mRNA is commonly used, for example in Drosophila, zebrafish, Xenopus and mouse, in both cell culture and model organisms
[92][93][94][95][158,159,160,161]. In addition to the use of non-viral systems, Ling and colleagues found effective and successful delivery using a viral system. These authors used mLP-CRISPR, a lentiviral system that delivers mRNA encoding one of the longest Cas9 proteins (SpCas9) and gRNA simultaneously. By targeting vascular endothelial growth factor A (Vegfa), it was found that with only a single sub injection-retinal of mLP-CRISPR in mouse models, 44% of Vegfa in retinal pigment epithelium was knocked out and the area of choroidal neovascularization was reduced by 63% without inducing off-target edits or anti-Cas9 immune responses
[96][45]. Although CRISPR-Cas9 is most used to control over-expression levels of a particular protein, Qiu and colleagues verified the knockdown of the Angiopoietin-like 3 (Angptl3) gene in a specific and efficient way using the system CRISPR-mRNA Cas9, which led to a significant reduction in serum Angptl3 protein, low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) levels in wild-type C57BL/6 mice
[97][162], presenting similar results to studies with antisense oligonucleotides
[98][163]. They also verified that the therapeutic effect of genome editing was stable for at least 100 days after the single dose administration
[97][162]. Wang and collaborators demonstrated that CRISPR/Cas9 mRNA-mediated gene editing technology allowed the simultaneous disruption of five genes in mouse embryonic stem cells (ES) with high efficiency, thus verifying that with Cas9 mRNA co-injection and sgRNAs targeting Tet1 and Tet2 in zygotes achieved mutations in both genes with an efficiency of 80% in mice with biallelic mutations
[93][159]. This discovery not only allows us to verify that the CRISPR/Cas9 technology allows mutations in several genes simultaneously, but it will also greatly accelerate the in vivo study of functionally redundant genes and epistatic gene interactions.
Since the discovery of CRISPR-Cas9, the CRISPR revolution has expanded beyond its original use as a genetic engineering tool. New Cas nucleases are being developed to enable faster and more accurate molecular diagnostic platforms for use with next-generation bio-sensing platforms. Abbott and co-workers showed, through bioinformatic analysis, that some different CRISPR-associated RNAs (crRNAs) were able to target over 92% of live influenza A virus strains and over 91% of all coronaviruses. This fact expands CRISPR-Cas13 systems applications beyond diagnostics, such as SHERLOCK, and live-cell RNA imaging
[99][164]. In this context, Cas 13 is an endonuclease that targets and binds sg RNA. Moreover, it demonstrates RNA cis-cleavage activity when activated by a target RNA in different model organisms
[100][165] and is more effective and specific than RNAi in mammalian cells
[101][102][166,167]. For this purpose, CRISPR-Cas13 has been proposed and used as a tool for SARS-CoV-2 detection
[103][168]. Furthermore, Blanchard and co-workers found that using CRISPR/Cas13a mRNA specific for highly conserved regions of influenza virus and SARS-CoV-2, efficiently degraded influenza RNA in lung tissue when administered after infection, while in hamsters, Cas13a reduced replication of SARS-CoV-2 and reduced the symptoms
[104][169]. Lastly, a promising example for CRISPR-Cas13 application is the study of Rashnonejad and co-workers against Facioscapulohumeral muscular dystrophy. They developed different Cas13b-gRNAs that target various Double Homeobox 4 (DUX4) mRNA parts and verified a decrease over 90% of DUX4 protein in treated cells. Moreover, cell viability was improved, as well as cell death prevention in vitro and in vivo
[105][170]. Overall, the applications of the CRISPR-Cas13 in diagnostics are of interest and will open up new avenues for their in vivo applications, such as RNA knockdown and editing.