Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Flávia Leme and Version 2 by Vivi Li.
Alismataceae is a family consisting of floating to emergent aquatic or marsh herbs with a worldwide distribution. Limnocharitaceae was merged with Alismataceae to form one family comprising 17 genera and ca. 100 species.
- Echinodorus
- Helanthium
- Hydrocleys
- Limnocharis
- ontogenesis
- Sagittaria
- secretory ducts
1. Introduction
Alismataceae is a family consisting of floating to emergent aquatic or marsh herbs with a worldwide distribution [1]. Limnocharitaceae was merged with Alismataceae to form one family comprising 17 genera [1][2][3][4][1,2,3,4] and ca. 100 species [5]. The family is known for including species with economic uses as food sources [6], ornamentals (aquarium plants) [6][7][6,7] and medicinals [6][7][6,7], as well as species for beekeeping [1][6][1,6]. The largest genera are Echinodorus and Sagittaria, both regularly used as aquarium and pond ornamental plants [1][6][1,6]. Leaves of Echinodorus grandiflorus (Cham. and Schltdl.) Micheli and E. macrophyllus (Kunth) Micheli are used for medicinal purposes [7][8][9][10][11][12][7,8,9,10,11,12]. Ethnobotanical investigations and clinical trials indicate that E. grandiflorus leaves have anti-hypertensive, anti-inflammatory, diuretic, and anti-arthritic properties [7][10][11][12][13][7,10,11,12,13].
Laticifers have been reported in some families of Alismatales [14], such as Aponogetonaceae [15], Araceae [16][17][16,17] and Alismataceae [14]. According to other authors, laticifers have not been recorded in Alismataceae [18][19][18,19] and Butomaceae [20], while resin secretory ducts have been described only for Araceae [14].
The secretory structures of Alismataceae have been described as secretory ducts, laticifer ducts or laticifer canals [1][19][20][21][22][23][24][1,19,20,21,22,23,24], and the secretion is described as latex [23] or milky juice [1]. However, laticifer ducts or laticifer canals are not found in the specialized literature on secretory structures [25][26][27][25,26,27]. In addition, the lack of ontogenetic analyses for the exact classification of these structures does not allow the establishment of homology in the family or order [23].
The secretory ducts or canals are elongated secretory structures lined with an epithelium of live secretory cells delimiting a large intercellular space (lumen) [14][25][26][27][28][14,25,26,27,28]. Ontogenetically, they may develop by schizogeny (separation of cells), lysigeny (disintegration of cells) or both (separation and disintegration of cells) [25]. Their development occurs from a group of a few initial meristematic cells that form a rosette in cross-section. These cells undergo divisions in various directions, and the rosette becomes more distinct from the surrounding cells, constituting the future epithelium [14][25][27][29][14,25,27,29]. The lumen develops in the middle of the epithelium, and further periclinal divisions may occur outside the epithelium, forming a sheath with one or more cell layers. The material secreted by the duct varies between resin, gum or mucilage [14][25][26][29][14,25,26,29].
Laticifers are a specialized type of secretory structure with an emulsion or suspension of compounds of a varied nature, in which terpenoids predominate, called “latex” [25][26][27][28][30][31][25,26,27,28,30,31]. The latex color may vary according to composition; it may be white (milky) [32][33][32,33], yellow [30][34][30,34], orange [35], red [36] or colorless [34]. Laticifers consist of one cell with intrusive growth (nonarticulated non-anastomosing type) or a series of connected cells (articulated anastomosing type) that form a uniseriate tube [25][30][34][37][25,30,34,37]. Articulated anastomosing laticifers or nonarticulated laticifers can be branched or unbranched [25][26][27][30][34][37][25,26,27,30,34,37]. Articulated anastomosing laticifers in a mature phase have terminal walls that disintegrate (multinucleated structure), that can branch and can assume several forms [25][30][34][37][25,30,34,37].
Laticifers and secretory ducts have been cited in at least 40 and 50 families, respectively, of vascular plants, including phylogenetically unrelated plants such as ferns, gymnosperms, and angiosperms, and they have emerged many times in the course of plant evolution [14][30][38][14,30,38]. Laticifers and secretory ducts have roles in herbivory reduction or resistance in plants. Their products (latex–laticifers; resin, gum or mucilage–secretory ducts) are of great economic importance since they are crucial for the production of pharmaceuticals, enzymes and rubber [28][31][39][28,31,39].
Although there are several anatomical studies on Alismataceae species [19] such as Echinodorus spp. [23][24][23,24], Helanthium tenellum [7], Alisma plantago [40], Echinodorus macrophyllus [41] and Sagittaria montevidensis [22], these studies do not provide details regarding the anatomy and ontogeny of the secretory structures called the “laticifer ducts” [1][11][14][22][23][24][1,11,14,22,23,24] or secretory canals [41]. Histochemical studies on the composition of the secretion are also absent. Thus, there is nothing to indicate whether the secreted material has latex or resin characteristics. Therefore, our objective was to study the structure, ontogeny, distribution in the organs and secretion composition of the secretory structures present in Echinodorus grandiflorus, H. tenellum, Hydrocleys nymphoides, Limnocharis flava and Sagittaria rhombifolia in order to determine whether the analyzed species have laticifers or secretory ducts.
2. Current Researchaes and Results
In E. grandiflorus, H. tenellum, H. nymphoides, L. flava and S. rhombifolia (Figure 1A–F), we found elongated secretory ducts consisting of an intercellular space or lumen lined with one layer of secretory cells, i.e., the epithelium. The lumen, where the secretion is released and stored, is formed by schizogeny during organ development (Figure 2A–F and Figure 3A–H). In the species evaluated, the secretory ducts are extremely narrow, with a diameter ranging from 11.9 µm in H. tenellum to 41.8 µm in H. nymphoides.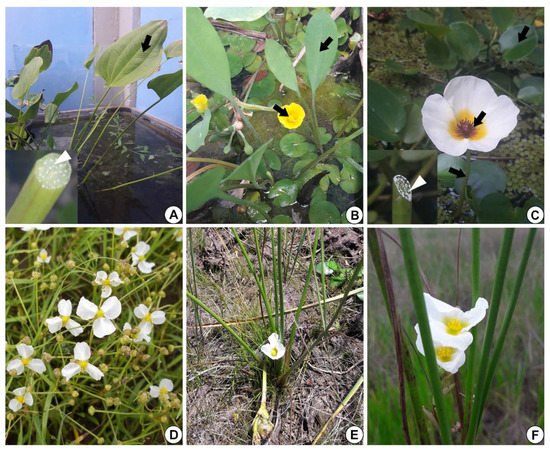
Figure 1. Alismataceae species analyzed. (A) Echinodorus grandiflorus leaf (arrow). Inset: detail of the petiole with white secretion (white arrowhead). (B) Limnocharis flava showing leaves and flowers (arrows). (C) Hydrocleys nymphoides: leaf, petiole and flower (arrows). Inset: note on the left side the petiole with white secretion (white arrowhead). (D) Helanthium tenellum (Image D: Giseli Catian). (E,F) Sagittaria rhombifolia.
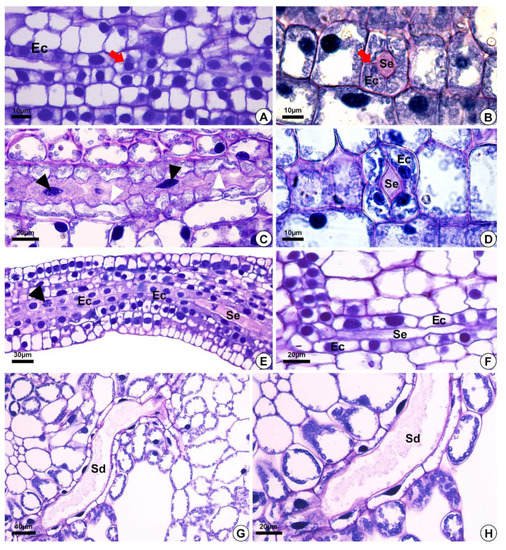
Figure 2. Light micrographs after staining with toluidine blue, depicting the development of secretory ducts in Helanthium tenellum, Hydrocleys nymphoides, Limnocharis flava and Sagittaria rhombifolia. (A,F) Hydrocleys nymphoides. (B–D) Limnocharis flava. (E) Helanthium tenellum. (G,H) Sagittaria rhombifolia. Longitudinal (A,C,E,F) and cross-section (B,D) of the floral meristem. (A,B) Secretory ducts originated from the ground meristem by asymmetrical mitotic divisions (red arrow) (A). The initial epithelial cells start to pull away (schizogeny) and give rise to the lumen (B). (C) Secretory duct development. Note epithelial cells with fusiform nuclei (black arrowheads), and an undulating cell wall (white arrowheads). (D–F) Epithelial cells with large nuclei and dense cytoplasm. (E) Epithelial cells in division (arrowhead), with an increase in the number of secretory cells around the lumen. (G,H) Mature secretory ducts. (G) Branching of secretory ducts by anastomosis. (H) Note the presence of secretion. Sd = secretory ducts. Ec = epithelial cells. Se = secretion.
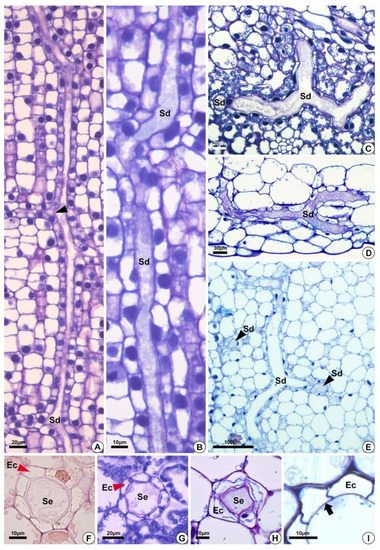
Figure 3. Structure of the secretory ducts of Hydrocleys nymphoides (A,B), Sagittaria rhombifolia (C,F,G), Limnocharis flava (D,E,H), and Echinodorus grandiflorus (I). (A–E) Longitudinal sections. (F–I) Cross-sections showing secretory ducts in the aerenchyma tissue of the petiole. All preparations were stained with toluidine blue, except for the one shown in (F), which was stained with Schiff reagent (PAS). (A) Secretory ducts with anastomoses in different directions (black arrowhead). (B) Elongated secretory ducts. (C–E) Anastomosing secretory ducts “Y”-shaped. (D) Epithelial cells surrounding the lumen. (E) Secretory ducts in longitudinal (Sd) and cross-sections (black arrowheads–Sd). (F–H) Cross-section showing secretory ducts with a layer of epithelium (red arrowheads) composed of variable epithelial cell numbers. (I) Pectocellulosic cell wall of epithelial cells (arrows). Sd = secretory ducts. Ec = epithelial cells. Se = secretion.
2.1. Origin and Morphology of the Secretory Structures
The secretory ducts of E. grandiflorus, H. tenellum, H. nymphoides, L. flava and S. rhombifolia differentiate early during organ development, being fully visible while other tissues are still meristematic (Figure 2A–E). Thus, the secretory ducts are formed before the complete development of the surrounding tissues. Secretory ducts originate from the ground meristem (Figure 2A–E) by asymmetrical mitotic divisions, followed by the dissolution of the middle lamella between the cells, which gives rise to the lumen in the early stage of development when the epithelium is composed of only four cells (Figure 2B–D). Initially, the cells of the rosette have large nuclei and a dense and uniform cytoplasm. These initial cells are distinguished from the adjacent meristematic cells by asymmetrical cell divisions (Figure 2A,B), dense cytoplasm and fusiform nuclei (Figure 2C). The wall of the epithelial cells becomes progressively undulating in L. flava (Figure 2C); however, in S. rhombifolia, the cell wall is smooth throughout development (Figure 2G,H). The ducts branch through lateral anastomoses with other secretory ducts (Figure 2G). At maturity, most ducts form an interconnected network of canals that extend longitudinally and radially throughout the shoot system (Figure 3A–E). Branched ducts are easily recognized by specific shapes such as a Y-bifurcation pattern (Figure 3C–E).
All species analyzed have ducts with a single-layered epithelium (Figure 3F–H). However, the epithelial cells are morphologically variable in length and width. They are large in H. tenellum and H. nymphoides (Figure 3A,B) but small and narrow in E. grandiflorus, S. rhombifolia and L. flava (Figure 3C–E). In mature secretory ducts, the epithelium is composed of 5 to 8 cells (Figure 3F–H) as seen in cross-section in all species analyzed. Epithelial cells generally have a flattened shape and thin cell walls that project into the lumen, have a slightly dense cytoplasm and large nuclei and contain small plastids. (Figure 3). The cell wall is pectocellulosic, reacting positively with Schiff’s reagent (PAS) (Figure 3F) and staining magenta with toluidine blue (Figure 3H,I).
2.2. Distribution of the Secretory Ducts in the Plant
Secretory ducts are present in the leaves (petiole, blade and midrib) of the five species analyzed and in the flowers of H. nymphoides, L. flava, and S. rhombifolia (Table 1, Figure 4). They occur at higher frequency in H. nymphoides, L. flava, and S. rhombifolia and at lower frequency in H. tenellum and E. grandiflorus. In the five species studied, secretory ducts were found in the petiole (Figure 4A), leaf blade, and midrib (Figure 4B,C). In H. nymphoides, L. flava, and S. rhombifolia flowers, the secretory ducts were distributed in the perianth and stamens (Figure 4D–F). The secretory ducts occur in the subepidermal layer, mesophyll, aerenchyma tissues and diaphragm (Figure 4). In the aerenchyma, they are distributed around the vascular bundles and pass among the aerenchyma spaces (Figure 4G–I).
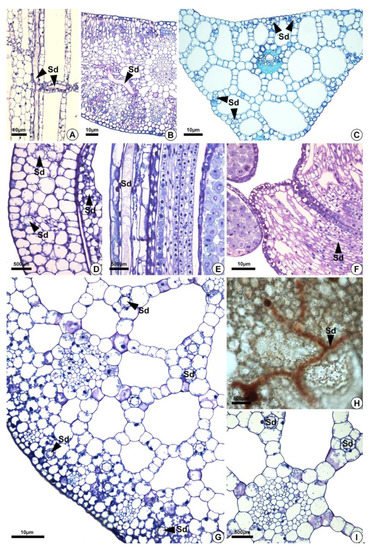

Figure 4. Distribution of secretory ducts in the petiole, leaf blade, and perianth of Sagittaria rhombifolia and Helanthium tenellum. (A,B,D–I) Sagittaria rhombifolia. (C) Helanthium tenellum. All preparations were stained with toluidine blue, except for (H) which was stained with oil red reagent that reacted positively in orange for lipids. (A) Longitudinal section of the petiole. Note the secretory ducts distributed vertically and longitudinally in the aerenchyma (arrowheads). (B,C) Secretory ducts in the leaf blade and the midrib of a cross-section. (D–F) Longitudinal section of the floral organs. (D,E) Secretory ducts in the perianth (arrowheads). (F) Secretory ducts in the filament (arrowheads). (G–I) Cross-sections. (G) Cross-section of a petiole. Note the secretory ducts in the cortex and the aerenchyma (arrowheads). (H) Secretory ducts in the diaphragm showing lipids in orange. (I) Secretory ducts in the aerenchyma around the vascular bundle. Sd = secretory ducts.
Table 1. Distribution of secretory ducts in the vegetative and floral organs of Echinodorus grandiflorus, Helanthium tenellum, Hydrocleys nymphoides, Limnocharis flava and Sagittaria rhombifolia. Symbols: (+) presence; (−) absence; (NA) not analyzed.
| Organ | Echinodorus grandiflorus | Helanthium tenellum | Hydrocleys nymphoides | Limnocharis flava | Sagittaria rhombifolia | |
|---|---|---|---|---|---|---|
| Leaf | Petiole | + | + | + | + | + |
| Blade | + | + | + | + | + | |
| Flower | Floral scape | NA | NA | + | + | + |
| Floral organs | NA | NA | + | + | + |
2.3. Secretion Composition
The secretion in the leaves and petioles of E. grandiflorus, H. tenellum, H. nymphoides, L. flava and S. rhombifolia was milky (Figure 1A,C). Compared to the other species analyzed, H. nymphoides showed the greatest exudation of secretion. The secretion was initially fluid and became thicker after air contact. The histochemical tests were performed on the petiole of the leaves (Figure 5A–K,P) or on floral buds (Figure 5L–O). The results demonstrated that the secretion produced by the secretory ducts was of resin and consisted mainly of lipids (Figure 5C–G), alkaloids (Figure 5H–J), proteins (Figure 5K,L) and polysaccharides (Figure 5M–O) (Table 2). The analysis showed that the secretion was white in fresh material (Figure 1A,C) and dark on the slide (Figure 5A,B). For lipids, the Sudan and the oil red tests were positive in all species of Alismataceae analyzed (Figure 5E–G). We also detected alkaloids (Figure 5H–J), proteins (Figure 5K,L), and polysaccharides (Figure 5M–P) including mucilage (Figure 5M,N,P) in the secretory ducts. Polysaccharides were weakly stained (Figure 5O). No phenolic compounds were found (Table 2).
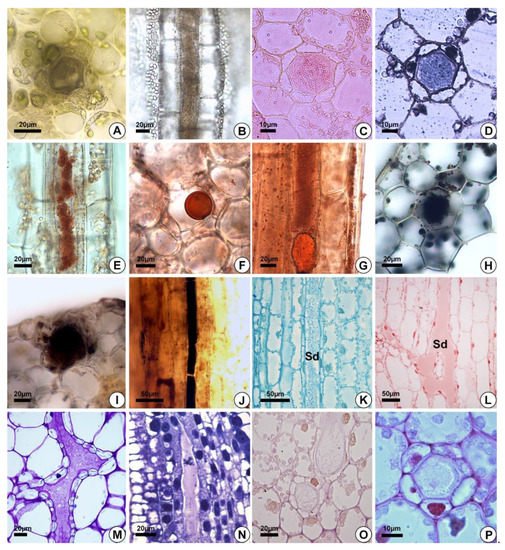

Figure 5. Histochemical analyses of the secretion. (A,F,G,L,P) Hydrocleys nymphoides. (B–E,K,O) Sagittaria rhombifolia. (J,N) Helanthium tenellum. (I,M) Limnocharis flava. (H) Echinodorus grandiflorus. (A,B) Secretory ducts without staining. (C–G) Positive reaction of the secretion for total lipids (Sudan IV (C), Sudan black (D) and oil red (E–G)). (H–J) Alkaloids (Wagner’s reagent). (K,L) Proteins (Coomassie blue (K) and xylidine Ponceau (L)). (M,N) Secretion with acidic substances (toluidine blue). (O) Neutral polysaccharides (PAS). (P) Positive reaction of the secretion for acidic mucilage (Ruthenium red). Sd = secretory ducts.
Table 2. Histochemical data obtained for the secretory duct secretion of Echinodorus grandiflorus, Helanthium tenellum, Hydrocleys nymphoides, Limnocharis flava and Sagittaria rhombifolia. Symbols: (+) presence; (−) absence.
| Reagent | Target Compound | Color | Echinodorus grandiflorus | Helanthium tenellum | Hydrocleys nymphoides | Limnocharis flava | Sagittaria rhombifolia |
|---|---|---|---|---|---|---|---|
| Schiff (PAS) | Neutral polysaccharides | pink | + | − | + | + | + |
| Lugol | Starch grains | blue | − | − | − | − | − |
| Sudan Black B | Total lipids | black | + | + | + | + | + |
| Sudan IV | Total lipids | light orange | + | + | + | + | + |
| Oil Red | Lipids | orange | + | + | + | + | + |
| Comassie Blue | Protein | blue | + | + | + | + | + |
| Wagner’s reagent | Alkaloids | blue-black to reddish | + | + | + | + | + |
| Toluidine Blue | Phenolic compounds | green | − | − | − | − | − |
| Ferric chloride | Phenolic compounds | brownish | − | − | − | − | − |
