C-reactive protein (CRP) is an acute-phase protein that is produced by hepatocytes and other cell types, including immune cells, endothelial cells, and smooth muscle cells, after stimulation of the gene encoding for CRP by Interleukin-6 (IL-6).
1. Role of CRP in Development of Schizophrenia
C-reactive protein (CRP) is an acute-phase protein that is produced by hepatocytes and other cell types, including immune cells, endothelial cells, and smooth muscle cells, after stimulation of the gene encoding for CRP by Interleukin-6 (IL-6) [38]. The circulating levels of CRP in serum rise and fall according to the inflammatory status of the body and therefore it is the most commonly used biomarker of systemic inflammation worldwide [38,39]. CRP is a standard laboratory exam and can be measured in the peripheral blood and analyzed in any clinical laboratory around the globe. Therefore, CRP has a very promising potential in being a clinical biomarker for psychiatric disorders [40]. The high-sensitivity CRP (hs-CRP) assay has a lower limit of detection of 0.1 mg/L. However, these detection limits may vary from manufacturer to manufacturer. The measurement of CRP is useful in the diagnosis and monitoring of many acute and chronic inflammatory conditions of both infectious and non-infectious etiology [41].
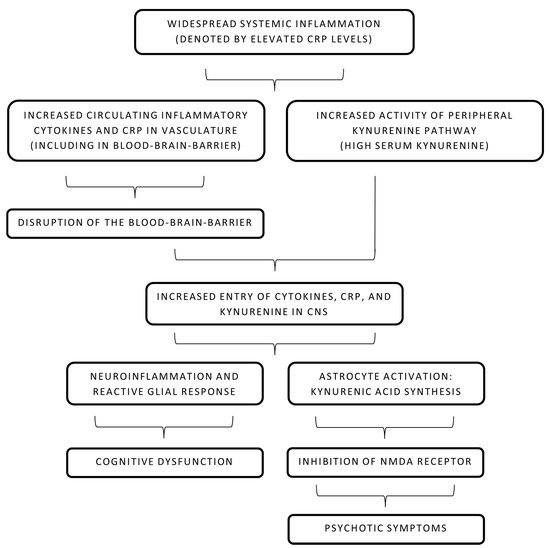
The role of CRP in the causality of schizophrenia has been a subject of interest for many years. It is known that CRP and other acute-phase reactants cause disruption of the BBB and alter its permeability for inflammatory mediators and antibodies [1][2]. As described above, this compromise in the integrity of the BBB in the backdrop of widespread systemic inflammation has been linked to developing psychotic symptoms [3][4]. Increased CRP has also been recently linked with a significant decline in multiple cognitive domains, including working memory and learning ability, in individuals of all ages suffering from schizophrenia [5]. Further reviews were consistent in highlighting a marked cognitive impairment in schizophrenic patients with even low-grade or subclinical inflammation [6][7]. Interestingly, a study in Finland elucidated the role of elevated maternal CRP levels in increasing the risk of psychosis in the offspring [8]. In summary, an inverse association of CRP levels and cognitive ability is noted in acute psychosis. However, a clinical trial published in 2019 provided valuable insight into the prognostic value of CRP in six months following the resolution of acute psychosis [9]. Cognitive abilities improved with time and a noticeable drop in CRP levels was noted in the later periods, continuing the inverse relationship [9]. The pathophysiology behind increased CRP levels and subsequent cognitive dysfunction is not fully known but some studies have implicated CRP in increasing BBB permeability during acute inflammatory phases and causing neuroinflammation [10]. Mouse models revealed that CRP has no virtual effect on BBB permeability in smaller amounts but impairs its function and increases paracellular permeability in higher amounts that may be achieved during high systemic inflammation or pro-inflammatory states like psychosis [10]. Multiple complex molecular pathways have been described to explain CRP-led increased permeability of the BBB and endothelial dysfunction [10]. Notably, it is widely believed that the disruption of tight junctions is involved in the increased permeation of CRP [2]. CRP in blood activates the surface Fc-gamma receptors (CD16/32) on endothelial cells and leads to the formation of reaction oxygen species (via p38-mitogen-activated protein kinase mechanism) that causes an alteration in the myosin light chain kinase activity [2]. This implies that impairment of tight junctions by CRP is most likely achieved via modification of the cytoskeletal structure and induction of abnormal contractility [2][10]. After entering the CNS, CRP causes a reactive microglial reaction, astrogliosis, and neuroinflammation [11]. Neuroinflammation in psychiatric conditions is invariably associated with short-term and lasting cognitive deficits [12]. Therefore, this mechanism may explain how elevated CRP levels (reflecting systemic inflammation) in psychosis may lead to decreased cognitive abilities.
The role of CRP in the causality of schizophrenia has been a subject of interest for many years. It is known that CRP and other acute-phase reactants cause disruption of the BBB and alter its permeability for inflammatory mediators and antibodies [42,43]. As described above, this compromise in the integrity of the BBB in the backdrop of widespread systemic inflammation has been linked to developing psychotic symptoms [15,25]. Increased CRP has also been recently linked with a significant decline in multiple cognitive domains, including working memory and learning ability, in individuals of all ages suffering from schizophrenia [44]. Further reviews were consistent in highlighting a marked cognitive impairment in schizophrenic patients with even low-grade or subclinical inflammation [45,46]. Interestingly, a study in Finland elucidated the role of elevated maternal CRP levels in increasing the risk of psychosis in the offspring [13]. In summary, an inverse association of CRP levels and cognitive ability is noted in acute psychosis. However, a clinical trial published in 2019 provided valuable insight into the prognostic value of CRP in six months following the resolution of acute psychosis [47]. Cognitive abilities improved with time and a noticeable drop in CRP levels was noted in the later periods, continuing the inverse relationship [47]. The pathophysiology behind increased CRP levels and subsequent cognitive dysfunction is not fully known but some studies have implicated CRP in increasing BBB permeability during acute inflammatory phases and causing neuroinflammation [48]. Mouse models revealed that CRP has no virtual effect on BBB permeability in smaller amounts but impairs its function and increases paracellular permeability in higher amounts that may be achieved during high systemic inflammation or pro-inflammatory states like psychosis [48]. Multiple complex molecular pathways have been described to explain CRP-led increased permeability of the BBB and endothelial dysfunction [48]. Notably, it is widely believed that the disruption of tight junctions is involved in the increased permeation of CRP [43]. CRP in blood activates the surface Fc-gamma receptors (CD16/32) on endothelial cells and leads to the formation of reaction oxygen species (via p38-mitogen-activated protein kinase mechanism) that causes an alteration in the myosin light chain kinase activity [43]. This implies that impairment of tight junctions by CRP is most likely achieved via modification of the cytoskeletal structure and induction of abnormal contractility [43,48]. After entering the CNS, CRP causes a reactive microglial reaction, astrogliosis, and neuroinflammation [49]. Neuroinflammation in psychiatric conditions is invariably associated with short-term and lasting cognitive deficits [50]. Therefore, this mechanism may explain how elevated CRP levels (reflecting systemic inflammation) in psychosis may lead to decreased cognitive abilities.
Apart from CRP’s direct role in the disruption of the BBB, its role in the increment of the kynurenine pathway is still unclear. High kynurenic acid levels, as described earlier in this text, act as an antagonist at the NMDA receptor and possibly contribute to psychotic symptoms [13]. Both CNS and peripheral kynurenine pathways are tightly regulated by the immune status of the body [14]. With increased BBB permeability in the setting of elevated CRP levels, infiltration of CRP and other cytokines in CNS leads to neuroinflammation and increased activation of astrocytes and microglia [15]. Tryptophan degeneration and kynurenic acid production are increased and the antagonistic effect on NMDA is more pronounced, leading to psychotic symptoms [14]. It is also important to consider that some studies have concluded that CRP levels are not associated with the kynurenine pathway or levels of kynurenic acid in CNS [16][17]. It is important to note that CRP levels have been largely associated with cognitive symptoms in psychosis, while kynurenic acid levels are associated with psychiatric symptoms [18]. Therefore, it is unclear whether CRP levels and kynurenic acid levels act as two independent markers occurring coincidentally during schizophrenia or have a complex interaction that leads to a possible cause–effect relationship.
Apart from CRP’s direct role in the disruption of the BBB, its role in the increment of the kynurenine pathway is still unclear. High kynurenic acid levels, as described earlier in this text, act as an antagonist at the NMDA receptor and possibly contribute to psychotic symptoms [28]. Both CNS and peripheral kynurenine pathways are tightly regulated by the immune status of the body [33]. With increased BBB permeability in the setting of elevated CRP levels, infiltration of CRP and other cytokines in CNS leads to neuroinflammation and increased activation of astrocytes and microglia [51]. Tryptophan degeneration and kynurenic acid production are increased and the antagonistic effect on NMDA is more pronounced, leading to psychotic symptoms [33]. It is also important to consider that some studies have concluded that CRP levels are not associated with the kynurenine pathway or levels of kynurenic acid in CNS [52,53]. It is important to note that CRP levels have been largely associated with cognitive symptoms in psychosis, while kynurenic acid levels are associated with psychiatric symptoms [54]. Therefore, it is unclear whether CRP levels and kynurenic acid levels act as two independent markers occurring coincidentally during schizophrenia or have a complex interaction that leads to a possible cause–effect relationship.
Figure 1. Pathophysiology of systemic inflammation and schizophrenia. CNS: central nervous system, CRP: C-reactive protein, NMDA: N-methyl-D-aspartate.
2. Associations of CRP with Psychotic Symptoms and Role in Clinical Evaluation
A review of current findings necessitates further research on a molecular level that illustrates the processes behind the appearance of psychotic symptoms in patients with high CRP levels. However, Mendelian randomization (MR) studies on CRP and risk of developing schizophrenia have mostly shown CRP to have a protective causal effect. Ligthart believes it is due to the conventionally accepted antimicrobial qualities of CRP that allow avoidance of childhood infections, which is one of the risk factors of developing psychosis
[19][55].
Apart from cognitive deficits, CRP levels have been associated with a variety of psychotic symptoms and different forms of schizophrenia. An association of CRP levels and the appearance of negative symptoms was also found via a cross-sectional study
[20][56]. Additionally, a recent systematic review recorded elevated CRP levels to be notably associated with positive symptoms of acute psychosis seen in schizophrenia
[21][39]. Furthermore, the findings showed an increased serum level of CRP may also be used to predict the onset of schizophrenia along with various cognitive and physical complications of schizophrenia
[21][39]. Inability to distinguish meaningful sensory stimuli from others, known as sensory gating deficit, has also been associated with elevated CRP levels in psychosis
[22][57]. However, it is important to note that findings of individual cross-sectional studies conducted to investigate the association of CRP and psychotic symptoms manifesting in schizophrenia show considerable heterogeneity and variable conclusions
[21][39]. Moreover, the findings of this systematic review by Fond et al. are inconclusive about the exact role of CRP
[21][39]. While an increased CRP level is noted, it is unclear whether this is an effect of schizophrenia or it is directly involved in the pathogenic mechanism behind it
[21][39].
However, it is crucial to note that the findings of the systemic review are in line with conclusions of an earlier meta-analysis consisting of 1963 patients with schizophrenia compared with 3683 non-schizophrenics
[23][58]. A modest rise in CRP levels was associated with schizophrenia in comparison to healthy controls
[23][58]. Another study analyzed baseline CRP levels and prevalence of schizophrenia (with possible hospitalization) in 78,810 Danish males
[1][42]. It found baseline CRP levels in individuals with schizophrenia were 63% higher compared to healthy individuals
[1][42]. A large meta-analysis consisting of 85,000 subjects also found a modest rise in CRP levels in patients with schizophrenia
[24][59]. Along with CRP, some studies have shown higher levels of baseline neutrophils and other innate immunological markers in schizophrenia
[4][25]. In addition, individuals with an elevated baseline CRP (due to any cause such as an autoimmune disease) level had a six to eleven times greater chance of developing schizophrenia late in their adult life
[1][42]. Further evidence from various geographical subgroups linking CRP levels with schizophrenia has strengthened the notion that there is an immune component in active and latent psychosis
[25][26][27][60,61,62].
A recent meta-analysis of 21 observational studies involving 7682 subjects demonstrated how CRP is associated with higher suicidality in patients with mental disorders
[28][63]. This could lead to an increase in our understanding of the pathophysiological underpinnings of suicide and improve its prevention
[28][63]. In light of continued identification of environmental and non-genetic risk factors of schizophrenia and psychosis, recognition of patients at “high risk” is becoming increasingly possible. Therefore, serum CRP levels, along with levels of other inflammatory mediators, in high-risk individuals have the potential of becoming a useful tool in predicting the appearance of psychotic symptoms
[5][44]. Furthermore, although scarce, evidence exists for elevated CRP levels being associated with increased mortality in various psychiatric disorders including psychosis
[29][64]. Despite encouraging advances in investigating immune molecules as a marker for predicting psychosis, it is still not conclusively known whether the prominent inflammation shown to coincide with schizophrenia occurs in the premorbid phase too
[30][65]. Currently, evidence found via meta-analyses is still inadequate to use CRP as an accurate predictor to determine the status of which high-risk individuals would “convert” into psychosis
[5][30][31][44,65,66].
3. CRP and Treatment of Schizophrenia
CRP has been used to assess response to treatment in many psychiatric and non-psychiatric conditions owing to its status as a relatively simple and readily attainable marker of peripheral inflammation
[32][67]. The available clinical data through individual longitudinal studies are largely heterogeneous with varying effects on CRP levels observed after treatment of schizophrenia was initiated via commonly used antipsychotics, such as haloperidol, risperidone, or clozapine
[24][59]. However, some authors review the literature and propose that typical (affecting mainly dopaminergic pathways) and atypical (affecting both dopaminergic and serotonergic pathways) antipsychotics both alleviate the symptoms of acute psychosis partially via reduction of the overall inflammatory state
[33][68]. The classic side effects of long-term conventional antipsychotic use include weight gain and metabolic syndrome and that has been posited to be the reason behind increased inflammation and CRP levels that override their partial anti-inflammatory effect
[34][69]. Fernandes et al. performed multiple meta-analyses on the available clinical data and reported that therapy with solely typical or atypical antipsychotic medication has not been found to reduce or bring about a significant change in CRP levels
[24][59]. This is perhaps owing to the immense importance given to the notion of monoaminergic neurotransmitter disbalance in the causality of schizophrenia
[11][49]. With more clinical data now reported on the inflammatory characteristic of psychosis, it is imperative to review our treatment approach.
The role of CRP in the psychopharmacology of schizophrenia must not be limited just to its position as a marker of recovery or treatment success subsequent to antipsychotic therapy. Early recognition of abnormal CRP levels and identification of high-risk patients may assist and guide therapeutic approaches, including targeted anti-inflammatory medications
[32][35][67,70]. Therefore, to counter high-grade inflammation denoted by CRP, the use of “add-on” anti-inflammatory pharmacological agents (like non-steroidal anti-inflammatory drugs, or NSAIDs) with traditional antipsychotics in the treatment of schizophrenia becomes necessary. Functionally, the difference among NSAIDs depends on their activity as a COX-1 or COX-2 inhibitor and their subsequent effect on kynurenic acid
[33][68]. Activation of the kynurenine pathway is an important inflammatory sequela that is denoted by a high CRP level peripherally. Models on COX-isoforms in mouse models have revealed that COX-1 inhibitors increase the kynurenic acid levels in the brain while COX-2 inhibitors reduce them
[36][71]. Due to the direct association of increased kynurenic acid in CNS with psychotic symptoms, pharmacological agents are utilized to achieve a reduced kynurenic acid level. Selective COX-2 inhibitors like parecoxib, meloxicam, among others have been shown to decrease brain kynurenic acid levels and possibly treat psychosis
[36][71]. Another drug, celecoxib (a COX-2 inhibitor), has also been investigated and trials reported a significantly improved outcome and better functioning cognitive status in groups that were treated with celecoxib and risperidone (antipsychotic) compared to placebo groups
[37][72]. However, other anti-inflammatory agents have been highlighted in improving schizophrenic symptoms. Clinical trials have suggested agents like acetylsalicylic acid (aspirin) and N-acetylcysteine (NAC) have the potential to reduce some of the debilitating symptoms of schizophrenia
[38][39][73,74].
Furthermore, a high CRP level in an individual may denote their resistance to antipsychotic treatment
[32][67]. An increasing body of evidence now posits treatment-resistant schizophrenia (TRS) has a distinct pathogenesis compared to treatment-responsive schizophrenia
[40][41][75,76]. This can be possibly due to the conventional treatment methods attempting to restore dopaminergic balance in the CNS while TRS is not shown to have a prominent dopamine-related pathology
[40][75]. On the other hand, chronic inflammation has been implicated in TRS. A French population study also found an association between TRS, and high levels of peripheral inflammation measured via increased CRP levels
[42][77]. Measuring CRP levels, therefore, may allow clinicians to identify patients at high risk for developing treatment resistance to antipsychotics and in turn identify a cohort that will be a suitable candidate for receiving anti-inflammatory medication
[32][67]. Naturally, this further paves way for anti-inflammatory medication being particularly useful in alleviating negative symptoms in individuals with TRS
[42][77].