The first report of bacterial membrane vesicles appeared in the mid-twentieth century
[1]. In this study, the protein exotoxin secreted by
Vibrio cholerae was shown to be resistant to proteases. Transmission electron microscope (TEM) analysis suggested that this exotoxin is located within spherical structures containing components of the bacterial cell envelope. These structures, detected in cell-free supernatants obtained from liquid bacterial cultures in the exponential growth phase
[2], were named membrane vesicles (MVs).
As enveloped structures, MVs have the characteristics of vectors that enable the transport of substances highly sensitive to environmental conditions. They protect proteins enclosed in their lumen against enzymatic decomposition, degradation related to low or high pH and oxidative stress conditions. Therefore, it is not surprising that, in addition to proteins acquiring of nutrients from the environment, pathogenic bacteria also use MVs to transport toxins that directly affect host cells and enzymes promoting bacterial colonization, facilitating the disruption of infected tissues and spreading of infection in the host. We provide examples of the best characterized bacterial virulence factors associated with MVs in Table 1. The enrichment of certain proteins in MVs, at a higher concentration than found in bacteria, suggests a degree of specification for MVs in toxic activity, polymer decomposition, antibiotic inactivation or metal ion sequestration. The small size of MVs (ranging from 20–250 nm in diameter)
[3] permits them to overcome epithelial barriers, such as the gut–blood barrier (GBB), and enter tissues that are not colonized by the bacteria producing them. The presence of surface antigens allows MVs to interact with cells of the host immune system, so that virulence factors they transport can modulate (induce or inhibit) the immune response. MVs can also act as “traps” for antibodies circulating in the inhabited tissue, or for bacteriophages in the natural environment. The great versatility of vesicles is the result of variation in their structure and composition. The secretion of active factors in this form is one of the most complex and diverse mechanisms of bacterial interaction with the environment and other cells
[4].
2. Structure of Membrane Vesicles (MVs) and Mechanisms of Secretion
The production of MVs (both extracellular and intracellular) has been observed in organisms from all three domains of life
[5]. Research on bacterial vesicles has been ongoing for over 60 years, but the mechanisms of their biogenesis are still not fully understood. Several vesicle types have been described in Gram-negative and Gram-positive bacteria. The MVs exhibit the membrane features of the originating bacteria and thus could indicate the nature of their cargos, such as proteins and nucleic acids (
Figure 1).
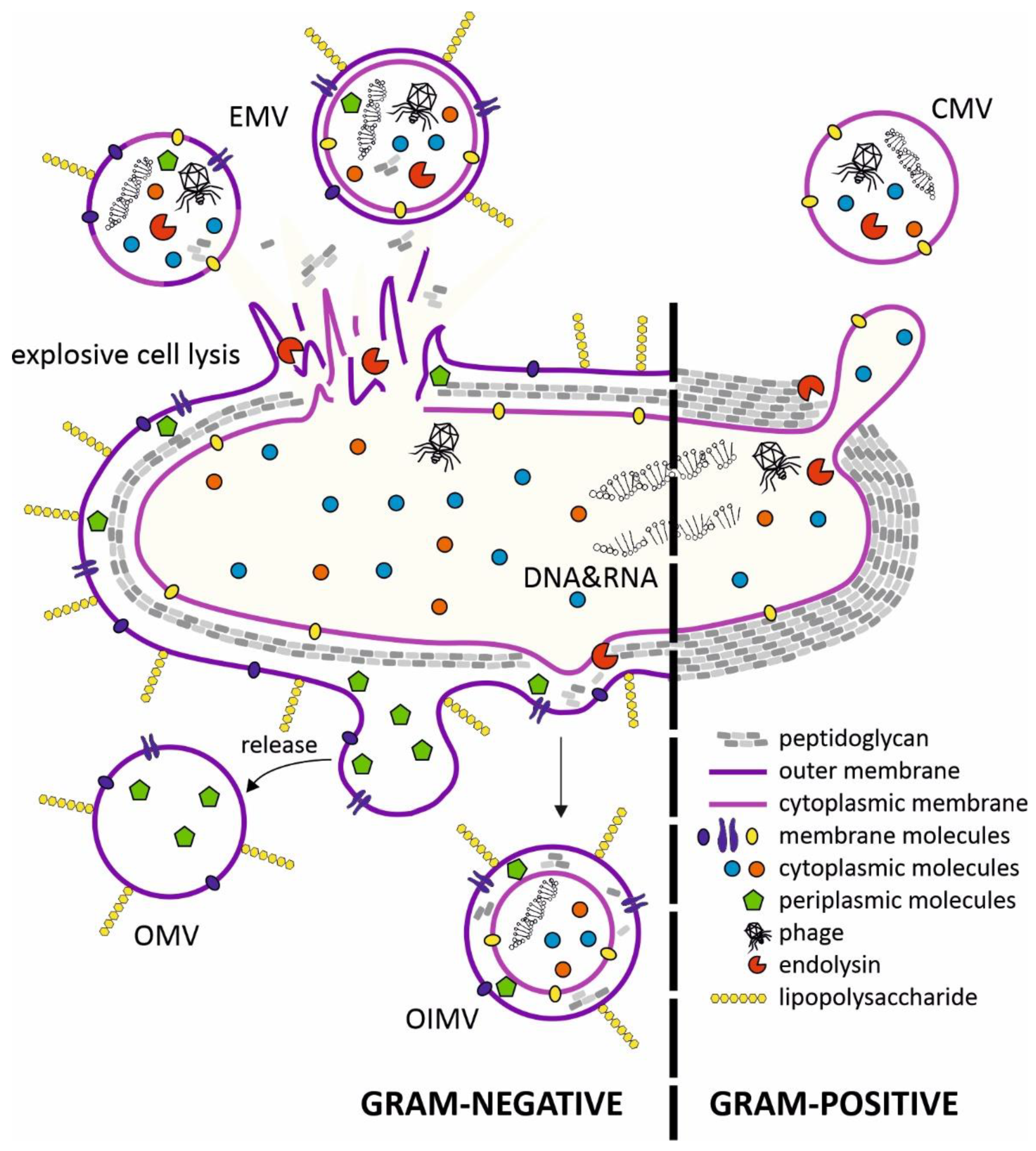
Figure 1. Mechanisms of bacterial membrane vesicle formation. In gram-negative bacteria, membrane vesicles are produced through membrane blebbing or explosive cell lysis triggered by phage-derived endolysins. Endolysins participate in the formation of cytoplasmic membrane vesicles (CMVs) in Gram-positive bacteria. The cytoplasmic membrane protrudes through holes in the peptidoglycan degraded by phage-derived endolysins. The contents of the membrane vesicles depends on the route of their formation. EMV—explosive membrane vesicle; OIMV—outer-inner membrane vesicle; OMV—outer membrane vesicle; CMV—cytoplasmic membrane vesicle.
OMVs (outer-membrane vesicles) produced by Gram-negative bacteria consist of blebs of bacterial outer membrane containing transmembrane proteins and LPS, with extracellular DNA (eDNA) exposed on the surface of OMVs, with periplasmic content packaged in the lumen of the vesicle. OMVs are produced by many species of pathogenic bacteria, including
Neisseria meningitidis,
Helicobacter pylori,
Escherichia coli (EHEC) and
Salmonella spp.
[6]. Increased secretion of OMVs usually occurs under stressful conditions, and is accompanied with the accumulation of misfolded proteins in the periplasm. According to one MV biogenesis model, the pressure of these defective proteins on the inner surface of the OM is responsible for bulging of the membrane and its detachment from the cell in the form of vesicles
[7].
Outer-inner membrane vesicles (OIMVs) are double-membrane structures and were first observed in cultures of
Pseudomonas aeruginosa [8]. The outer membrane and inner membrane are separated with a thin layer of periplasm with degraded fragments of peptidoglycan. The production of OIMVs are induced in stressful or adverse situations. Cytoplasm present in the lumen of these vesicles contains proteins and also fragments of DNA derived from the chromosome or plasmids and ATP
[8].
Vesicles containing cytoplasm are also produced by Gram-positive bacteria. The release of CMVs (cytoplasmic membrane vesicles) requires local peptidoglycan degradation by internal or external lytic enzymes (digesting both the glycan backbone and peptide bonds in the amino acid chains)
[9]. CMV production has been observed in several model Gram-positive bacteria, including
Bacillus subtilis [10],
Bacillus anthracis and
Staphylococcus aureus [11][12][11,12].
The last membrane vesicle type is EMVs (explosive membrane vesicles), which are the most diverse in terms of structure. They arise spontaneously during bacterial cell lysis. Fragments of membrane, together with the outflow of cytoplasm (also periplasm in the case of Gram-negative bacteria), create spherical membrane structures in the environment. Thus, the process of EMV “assembly” is cell-independent and spontaneous, so bacteria are unable to control the content of these vesicles. As a result, each lysing cell produces MVs that differ in size, composition and function. This process has been described in
P. aeruginosa biofilms, where deeply located cells subjected to hypoxia, nutrient deficiency and activation of the SOS system are autolysed through the activity of endolysins, and type R and F pyocins
[13]. EMVs released in this way are an important factor in the virulence of pathogenic
P. aeruginosa strains. As a component of the biofilm matrix, they bind eDNA and polysaccharides, and also bacteria via surface adhesins, which stiffens this structure
[14].
Several recent reviews describe the composition and biogenesis of bacterial membrane vesicles
[6][7][15][16][17][6,7,15,16,17]. In this article we present the latest data concerning interactions between MVs and selected human cell types.
3. Conclusions
The molecular understanding of bacterial virulence factors is an important challenge for microbiologists. Modern techniques have enabled the discovery of novel mechanisms that sometimes surprise researchers with their universality. This is the case for membrane vesicles, which play important roles in the interactions of bacteria with cells of other organisms. MVs are not only a new type of secretion system, their great variety of structure and function, action at a distance, and stability in the host system make them an important weapon in the bacterial arsenal. Examples of the best characterized bacterial virulence factors associated with MVs are presented below in
Table 1.
Table 1. Examples of bacteria producing membrane vesicles and active factors discovered inside/outside MVs.
| Bacterial Species (Gram-Negative) |
Active Factors |
Reference |
| Acholeplasma laidlawii PG8 |
-
adhesins/invasins—enable tight physical contact between bacterium and host cell
-
ABC transporting complexes
-
hydrolases: proteases, nucleases, and glycosylases
-
metallo-β-lactamase
|
[18][81] |
| Acinetobacter baumannii |
|
[19][82] |
| Actinobacillus pleuropneumoniae |
|
[20][83] |
| Aggregatibacter actinomycetemcomitans |
|
[21][84] |
| Bartonella henselae |
|
[22][85] |
| Borrelia burgdorferi |
|
[23][24][86,87] |
| Burkholderia cepacia |
|
[25][88] |
| Campylobacter jejuni |
|
[26][42] |
| Coxiella burnetti |
|
[27][89] |
| Escherichia coli K1 |
-
OmpA—interaction with Ecgp receptor on surface of brain microvascular endothelium leads to cell invasion; may also act in trans to promote cell invasion by other bacterial species
-
K1 antigen—polysaccharide antigen from cell envelope, linear polymer of NeuNac
-
TLR ligands—flagellin, lipoproteins, poly-CpG DNA strands
|
[28][90] |
Escherichia coli O157: H7
Shigella dysenteriae |
|
[29][91] |
| enterotoxic E. coli (ETEC) |
|
[30][92] |
| enterohemorrhagic E. coli (EHEC) |
|
[31][93] |
| extraintestinal pathogenic E. coli (ExPEC) |
|
[32][94] |
| Haemophilus influenzae type B (Hib) |
|
[33][95] |
| Legionella pneumophila |
|
[34][96] |
| Moraxella catarrhalis |
|
[35][97] |
| Neisseria meningitidis serogroup B |
|
[36][76] |
| Porphyromonas gingivalis |
-
gingipains—non-specific proteases degrading elements of host’s tissue and cytokines
-
HmuY—lipoprotein accumulating heme; assists biofilm formation process
-
factors assisting in co-localization with Treponema denticola
|
[37][98] |
| Salmonella enterica |
-
SopB—protects SCV (Salmonella-containing vacuoles) from degradation by reorganization of actin cytoskeleton
-
SipC—protein assisting in cell invasion process
-
SopA—ubiquitin ligase (E3) disturbing ubiquitin pathway of host cell
-
FljB—flagellin, strong antigen
-
SopE2—guanine nucleotide exchange factor (GEF); by catalysing exchange GDP → GTP disturbs function of Rho-protein family GTPases controlling dynamics of host cell cytoskeleton, which leads to membrane surface deformation and assists invasion process
-
PagK1/2—exact function still unknown; probably assists bacterial proliferation inside SCV
-
SrfN—promotes bacterial survival inside macrophages
|
[38][99] |
| Shigella flexneri |
|
[39][100] |
| Treponema denticola |
|
[40][101] |
| Vibrio cholerae |
|
[41][27] |
| Yersinia pestis |
-
Ail—surface adhesin; promotes contact with host cells
-
Pla—extracellular protease; activator of plasminogen
-
Caf1—fimbrial antigen F1; main component of OMVs
|
[42][102] |
| Bacterial Species (Gram-Positive) |
Active Factors |
Citations |
| Bacillus anthracis |
-
anthrolysin (ALO)—cholesterol-dependent cytolysin
-
lethal factor (LF)—zinc-protease; hydrolyses several MAPK-kinases (MAPKK), causes disturbance of signalling pathways and cell death
-
edema factor (ED)—calmodulin- and Ca2+-dependent adenylate cyclase; induces uncontrolled increase in cAMP concentration in phagocytic cells thus depleting ATP reserves
|
[ 12 ][12] |
| Clostridium perfringens |
|
[ 43 ][103] |
| Enterokok faeciumEnteroccoccus faecium |
-
fosfolipidy; zmniejszyć działanie przeciwbakteryjne antybiotyku daptomycynyphospholipids; reduce antibacterial activity of the antibiotic daptomycin
-
SdrD — białko wiążące kolagenSdrD—collagen-binding protein
-
PavA — białko wiążące fibronektynęPavA—fibronectin-binding protein
-
AtlA – autolizyna; wspomaga proces powstawania biofilmuAtlA—autolysin; assists in biofilm formation process
-
Acm — MSCRAMM (składniki powierzchni drobnoustrojów rozpoznające cząsteczki matrycy adhezyjnej) z grupy adhezyny; wiąże kolagenAcm—MSCRAMM (microbial surface components recognizing adhesive matrix molecules) group adhesin; binds collagen
-
Fnm — adhezyna wiążąca fibronektynęFnm—fibronectin-binding adhesin
-
PsaA – lipoproteina; potencjalny składnik przyszłej szczepionkiPsaA—lipoprotein; potential component of future vaccine
|
[ 44 ][104] |
| Prątek gruźlicyMycobacterium tuberculosis |
-
LpqH — lipoproteina; asystuje w procesach transportowychLpqH—lipoprotein; assists in transport processes
-
MPB83 — wysoce immunogenna glikoproteinaMPB83—highly immunogenic glycoprotein
-
LprA — lipoproteina; silny agonista TLR2LprA—lipoprotein; strong TLR2 agonist
-
PSTS3 — element systemu transportowego ABC związany z importem jonów fosforuPSTS3—component of ABC transport system connected with phosphorus ion import
-
lipoarabinomannan (LAM) — glikolipid powierzchniowy o właściwościach anty-ROSlipoarabinomannan (LAM)—surface glycolipid with anti-ROS features
-
mykobaktyna – powierzchnia Fe 3+ -syderoformycobactin—surface Fe3+-siderophore
|
[ 45 ][105] |
| Propionibacterium acnes |
|
[ 46 ][106] |
| Streptococcus mutans |
-
eDNA — ważny składnik biofilmueDNA—important biofilm component
-
glukozylotransferazy (GtfB/C/D) — wytwarzają adhezyjne zewnątrzkomórkowe polisacharydy z substratu sacharozyglucosyltransferases (GtfB/C/D)—produce adhesive extracellular polysaccharides from sucrose substrate
-
kwas lipotejchojowy (LTA) – antygen powierzchniowy; ważny w procesie adsorpcji w tworzeniu biofilmulipoteichoic acid (LTA)—surface antigen; important in adsorption process in biofilm formation
|
[ 47 ][107] |
| Streptococcus pneumoniae |
-
TatD — nieswoista DNaza umożliwiająca degradację NET (sieci DNA związane z białkami o działaniu przeciwdrobnoustrojowym: LL37, mieloperoksydaza, elastaza neutrofili)TatD—non-specific DNase enabling degradation of NETs (DNA nets associating with proteins with antimicrobial activities: LL37, myeloperoxidase, neutrophil elastase)
-
EndA — nieswoista DNaza zlokalizowana na powierzchni MVEndA—non-specific DNase located on surface of MVs
-
PspC-białko wiążące czynnik H; blokuje alternatywny szlak dopełniaczaPspC—H factor-binding protein; blocks alternative complement pathway
-
pneumolizyna (Ply) – egzotoksyna o właściwościach cytolitycznychpneumolysin (Ply)—exotoxin with cytolytic features
-
PsaA – adhezyna; silny antygen powierzchniowyPsaA—adhesin; strong surface antigen
-
SatA — transporter typu ABC; antygen powierzchniowySatA—ABC-type transporter; surface antigen
-
AmiA — białko wiążące peptydy; asystuje w aktywnym transporcieAmiA—peptide-binding protein; assists in active transport
-
MalX — białko wiążące maltozę i maltodekstrynęMalX—maltose and maltodextrin-binding protein
-
PnrA — transporter nukleozydów typu ABCPnrA—ABC-type nucleoside transporter
-
spr1909 — białko wiążące penicylinęspr1909—penicillin-binding protein
|
[ 48 ][108] |

