Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ekaterina Mikhailova and Version 3 by Dean Liu.
Gold nanoparticles (AuNPs) are extremely promising objects for solving a wide range of biomedical problems. The gold nanoparticles production by biological method (“green synthesis”) is eco-friendly and allows minimization of the amount of harmful chemical and toxic byproducts.
- gold nanoparticles
- antimicrobial activity
- anticancer activity
- antiviral activity
1. Antimicrobial Activity
Antibacterial activity. The high resistance of pathogenic microorganisms to different, and even the most modern antibiotics is becoming an increasingly serious problem for clinical medicine that could be decided using nanoparticles of various metals, including AuNPs. The antimicrobial activity is dependent on the method of synthesis, size, shape, and concentration of biosynthesized gold nanoparticles [1][183]. The influence mechanism for the pathogenic bacteria of the genus Bacillus, E. coli, Streptococcus, Staphylococcus, etc., is still extremely topical [2][3][4][103,106,184]. In addition, a significant point is the belonging of potentially destroyed bacteria to Gram+ or Gram−, according to their cell walls structural features. Although Gram-positive and Gram-negative cell walls are negatively charged with a high-affinity degree to positively charged AuNPs, having a thinner cell wall, Gram-negative bacteria are more simply exposed to AuNPs, while Gram-positive have rigid peptidoglycan layers on their surface, which prevent the AuNPs entry. For example, the inhibitory effect was shown only for Gram-negative bacteria in E. coli and Enterobacter ludwigii, B. subtilis, and Enterococcus faecalis research [5][39]. More considerable antibacterial effect was shown for bio-produced AuNPs compared with chemically synthesized gold nanoparticles [6][185]. Such antibacterial activity may be due to the synergistic effect of the plant compounds acting as capping agents [6][185]. AuNPs are a valuable element against bacterial biofilms. The AuNPs weaken the biofilm formation of Proteus sp. by inhibiting the production of virulence factors such as exopolysaccharides and metabolic activity such as surface hydrophobicity playing an important role in bacterium–host cell interactions and biofilm architecture in microbes, respectively [7][186]. In [8][187], bacterial surface attachment, flagella loss, biofilm assemblage, and clumping inside biofilm are demonstrated as the antibacterial processes.
Antifungal activity. Pathogenic fungi (C. albicans, Aspergillus spp., Penicillium spp., Trichoderma viridae, etc.) and their associated diseases represent a serious problem for clinical medicine. The emergence of new antibiotic-resistant strains requires the search for new methods of combating these pathogens. Among such potentially applicable substances, gold nanoparticles are emphasized. AuNPs interact with cell wall macromolecules, damaging them and affecting membrane proteins [9][188]. The inhibition of cell wall β-glucan synthase leads to changes in the cell wall integrity and further cell damage [9][10][188,189]. Besides, antifungal activity of gold nanoparticles is possible by increasing the ROS (for instance, in C. albicans) [10][189]. High antifungal activity was observed against C. tropicalis, C. albicans [11][190], A. flavus and A. terreus [12][191], A. fumigatus [13][192].
2. Antiviral Activity
Viral diseases have always posed the greatest of human threats. Notwithstanding that the investigation of these infectious agents is very intensive, we still know very little about combating methods. Moreover, for many known viral diseases, neither drugs nor vaccines were not found. Therefore, the struggle methods search against these extremely dangerous organisms stays a very urgent task requiring a prompt, and sometimes immediate decision. Metal nanoparticles are a very promising trend in fighting against various kinds of viruses. It is supposed that AuNPs can bind to a viral particle, blocking the connection with cellular receptors or viral receptors that inhibit viral cycle onset [14][193]. Aside from that, nanoparticles adsorbed on the cell surface can significantly change the membrane potential, leading to blocking the viral penetration into the cell [14][193]. Additionally, the inhibition of virus binding and penetration into the host cell, binding to the plasma membrane, inactivation of viral particles before penetration, and interaction with double-stranded DNA were found to be the antiviral mechanism of AuNPs [14][193]. For instance, gold nanoparticles are offered as an innovative means to counteract the measles virus (MeV) [15][194]. The active inhibition evidence of MeV replication in Vero cells by AuNPs obtained from garlic extract (Allium sativa) was discovered [15][194]. The interaction of AuNPs and MeV is probably resulting in the viral receptors blocking, preventing cell adsorption and the viral infection onset in the host cell. This type seems to be an ideal antiviral approach that excludes interaction with the cell. Additionally, having high stability and biocompatibility, AuNPs can easily interact with various biologically active compounds of garlic extract, including organosulfur compounds, saponins, phenolic compounds, and polysaccharides [15][194]. The active components are garlic organosulfur compounds, such as allicin, and products derived from allicin (diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, allyl-cysteine, and allyl-cysteine sulfoxide), which gives additional positive features against viral infection [16][195]. El-Sheikh et al. identified that AuNPs inhibited the replication of the Herpes Simplex (HSV-1) virus infection to Vero cells in a dose-dependent manner which reduced 90% CPE of HSV-1 at 31.25 μL [17][196]. Gold nanoparticles synthesized in Sargassum wightii extract prevented HSV-1 and HSV-2 viruses’ infection of Vero cells in a dose-dependent manner; moreover, the toxicity absence in high concentrations makes these AuNPs a potential antiviral agent [18][197]. However, there are other data regarding the gold nanoparticle’s effect on the vital activity of viruses: AuNPs can penetrate through the cell membrane into cells, and then inhibit viral DNA and RNA replication [14][193]. For example, AuNPs inhibit post-entry Foot-and-Mouth Disease (FMD) virus replication, accompanied by the onset of intracellular viral RNA synthesis, while at non-cytotoxic concentrations, AuNPs do not exhibit extracellular viricidal activity and inhibition of FMD growth in infection early stages, including attachment and penetration [19][198]. Thus, the proposed mechanism of antiviral activity based on [14][15][16][17][18][19][193,194,195,196,197,198] was demonstrated in Figure 14. Unfortunately, data on the “green” synthesis of gold nanoparticles with antiviral effects are very poor. Most of the works are devoted to chemically produced functional nanoparticles modified with specific molecules. Such complexes can be the basis for drugs’ targeted delivery to organs and tissues, including antiviral fighting.
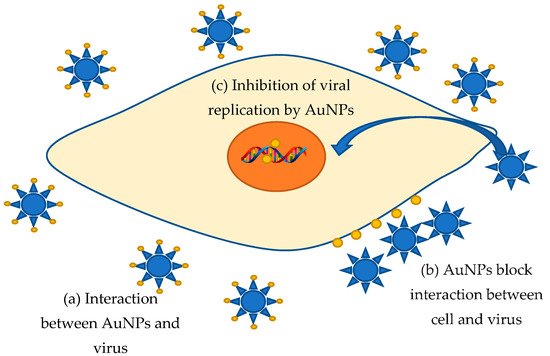
Figure 14. Proposal mechanism of AuNPs antiviral activity.
3. Antioxidant Activity
Different pathological conditions, including inflammatory processes, atherosclerosis, aging, cancer, and neurodegenerative diseases are highly dependent on oxidative stress caused by ROS, such as hydroxyl, epoxyl, peroxylnitrile, superoxide, and singlet oxygen. The redundant ROS amount or oxidative stress are influencing the host antioxidant system results in nucleic acid damage and enzyme inactivation [20][199]. Intracellular antioxidant enzymes and intake of antioxidants may help to maintain an adequate antioxidant status in the body [21][200]. Antioxidants help to reduce DNA damage, malignant transformation, cell damage, and decrease the risk of various pathologies. Antioxidants can decrease oxidative damage directly via reacting with free radicals or indirectly by inhibiting the activity or expression of free radical-generating enzymes or the activity increase or expression of intracellular antioxidant enzymes [21][200]. The antioxidant activity mechanism includes the following: the antioxidant molecules may directly react with the reactive radicals and destroy them, while they may become new less active free radicals, longer lived, and less dangerous than those radicals they have neutralized [21][200]. The search for new, safe compounds preventing oxidative damage is extremely meaningful, because despite the presence of effective endogenous antioxidant mechanisms in the human body, the balance between antioxidant action and free radicals’ production is disrupted because of lifestyle changes, radiation, and pollutants. The antioxidant potential of AuNPs produced by “green” synthesis is promising. The widely used and rapid methods for estimating antioxidant activity are the ABTS (2,2-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid radical) and DPPH (1,1-diphenyl–2–picrylhydrazyl radical) assays [22][201]. The free radical scavenging activity in vitro was shown for gold nanoparticles produced using extra virgin olive oil [23][202], nanoparticles synthesized from leaf extract (decoction) of Antigonon leptopus [24][203], Nerium oleander leaves extract [25][204], Kokum fruit extract [26][205], Cannonball fruit (Couroupita guianensis) extract [27][206], fruit extract of Hovenia dulcis [28][207], Aconitum toxicum rhizomes extracts [29][208], Artemisia capillaris, Portulaca oleracea, and Prunella vulgaris extracts [30][209], roots of Angelica pubescens [31][210], Thyme extract [32][211], leaves extract of Origanum vulgare [33][212], Piper longum fruit extract [34][213], marine bacterium Paracoccus haeundaensis [35][214], and others. According to most studies, various biomolecules encrusted on the surface of gold nanoparticles increase antioxidant activity. Especially polyphenols: flavins and flavonoids, as well as tannins, being powerful antioxidants themselves, enhance the effect [36][37][29][72,82,208].
4. Anticancer Activity
The last hundred years were marked by a huge increase in cancers, considered one of the main reasons for death worldwide. Unfortunately, most of the developed drugs and approaches have many side effects. Therefore, the new drugs with low toxicity and synthesized in a “green” way are very prospective anticancer agents. The antitumor effect of gold nanoparticles in vitro was shown for Hela N (Human cervix carcinoma), Hep G2 (human liver cancer), A549 (human lung carcinoma), MCF-7 (breast adenocarcinoma), HCT-11 (colon carcinoma), PANC-1 (human pancreatic cancer), ovarian adenocarcinoma (Caov-4) in a dose-dependent manner [38][39][40][41][42][43][44][215,216,217,218,219,220,221]. The provided gold nanoparticles effect depending on the shape, size, and chemical composition of the nanoparticle’s surface was discovered in [3][45][46][106,222,223]. Apparently, smaller gold nanoparticles have more antitumor effect due to the larger surface area of smaller NPs [47][224]. Undoubtedly, capping agents contribute to the antiproliferative activity of AuNPs, participating in the protein’s modification or cell growth enzymes and independently performing anticancer activity [48][47][49][79,224,225]. In addition, the antitumor activity of medicinal plant extracts is expressed by stopping the cell cycle, cell apoptosis, and induction of antiangiogenesis [50][51][226,227]. In this way, the plant-synthesized adsorbed active molecules and their therapeutic activity, as well as biocompatible gold nanoparticles are of great importance in anticancer therapy [49][225]. Although the mechanism of AuNP’s effect on cancer cells is not completely clear, the centerpieces are (a) ROS generation, (b) Glutathione (GSH) oxidation, (c) cell cycle arrest, and (d) caspases [52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141].
The AuNPs’ cytotoxic effect on cancer cells is primarily due to their easy permeability to cellular barriers and strong affinity for various biological macromolecules. As byproducts of normal cellular metabolism, ROS play an important role in cellular signaling pathways such as cell-to-cell signaling, cellular metabolism, cell proliferation, and cell apoptosis. The imbalance in ROS and antioxidant levels plays a critical role in tumor initiation and progression [69][228]. Gold nanoparticles can induce cytotoxicity through ROS, generating damage to cellular components through intracellular oxidative stress [70][71][229,230]. For example, AuNPs increase the ROS production in HeLa cells and probably lead to apoptotic cell death via the mitochondrial-mediated pathway [70][229]. Decreased mitochondrial membrane permeability and mitochondrial dysfunction leading to apoptosis were discovered for two human renal carcinoma cell lines [69][228].
Possessing antioxidant properties, GSH not only protects the cell from toxic free radicals but also generally determines the redox characteristics of the intracellular environment. It was found that ROS generation converts GSH to GSSG (Glutathione disulfide) through the oxidation process [72][231]. Oxidized glutathione is reduced by the enzyme glutathione reductase induced by oxidative stress. The ratio of reduced and oxidized glutathione forms in the cell is one of the most important parameters showing the oxidative stress level. For instance, low GSH levels were observed in cells influenced by star anise-synthesized AuNP [73][232]. A decrease in the GSH level corresponds to increased oxidative stress [73][232]. ROS generation in AuNPs-treated cells was also determined in other publications: increased oxidative stress and lipid peroxidation in MRC-5 (human lung fibroblasts); hydrogen-peroxide induced by GSH depletion is generated in HL7702 cells (human liver cell line) [74][75][233,234]. Thus, increasing ROS generation and glutathione oxidation may be the basis of AuNPs’ anticancer activity.
Physicochemical interactions of gold atoms with functional groups of intracellular proteins, as well as with nitrogenous bases and phosphate groups in DNA, are another cytotoxic action of gold nanoparticles [76][235]. The AuNPs influence various cell lines, for example, U87 (human primary glioblastoma cell line) is revealed in DNA degradation, condensed nuclei with fragmented chromatin structure [77][78][236,237]. Moreover, the formation of oligo-nucleosomal DNA fragments or ladder owing to DNA fragmentation is widely discussed as a biochemical marker of late apoptosis [79][238]. Another aspect is the accumulation of AuNPs-treated cells in the sub-G1 phase or G0/G1 phase of the cell cycle, so cell cycle regulation can play a vital role in the apoptosis induction [80][239]. Thus, a significant percentage of MCF-7 and MDA-MB-231 cells treated by “green” AuNPs were in the G0/G1 and S phases, which may indicate AuNP’s efficiency in inducing cell arrest at various phases of the cell cycle [78][81][237,240]. The launch of the apoptosis process–programmed cell death is one of the most important mechanisms of the antitumor effect. It is characterized by morphological changes: cell shrinkage, nuclei fragmentation, and extensive blebbing of the plasma membrane, eventually resulting in apoptotic cells formation that will subsequently be phagocytosed by macrophages [82][241]. Bcl-2 protein plays an essential role in the apoptosis process, which activates caspase-9 and caspase-3, triggering the apoptosis cascade (with the participation of another caspases-7,8) [83][242]. Besides, downregulation of p53 (protein p53) may also be a key element of anticancer activity, because it is a transcription factor regulating cell cycle and acting as a suppressor of malignant tumors formation [84][243]. AuNPs were demonstrated to induce the expression of both p53 and p21 in a concentration-dependent manner in MCF-7 [78][237]. Thereby, gold nanoparticles are capable of activating cell death through a caspase-mediated apoptotic pathway [85][86][87][88][244,245,246,247]. Nevertheless, there are still many questions about the anticancer activity of AuNPs; in addition, most studies were made in vitro and need further testing in vivo.
5. Other Activities
It should be noted that gold nanoparticles have other very useful properties.
Anti-inflammatory activity. One of the interesting AuNPs areas is using for anti-inflammatory activity. As mentioned earlier, ROS plays an important role in the activation of many inflammatory mechanisms. That is why gold nanoparticles inhibiting active oxidants are extremely promising in this field. Macrophages play an essential role in the development of inflammatory processes such as phagocytes [89][248]. LPS-induced RAW 264.7 macrophages are widely used as an in vitro model of inflammation [90][249]. Thus, the AuNPs influence the expression of iNOS (Inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) protein in LPS-induced (lipopolysaccharides-induced) RAW 264.7 cells for Acanthopanacis cortex extract was determined [91][250]. AuNPs produced using Panax ginseng fresh leaf extract exerted anti-inflammatory effects in LPS-induced RAW 264.7 macrophages by blocking NF-kB signaling (abnormal regulation of NF-kB activity can result in different diseases including inflammatory, cancer, metabolic, and cardiovascular illness) [92][251].
Antidiabetic activity. Despite the World Health Organization regularly developing norms and standards for diabetes diagnosis, treatment, monitoring, and its risk factors, the number of diagnosed cases is constantly increasing from year to year. The conducted experiments demonstrated AuNPs’ possibility to have an antidiabetic effect. Thus, oral AuNPs injection to diabetic animals regulates the metabolic process and restores cholesterol and triglycerides levels to almost normal levels [93][252]. Rats treated with gold nanoparticles were able to improve body weight by increasing insulin secretion and glycemic control, as well as due to their natural growth [94][253]. The glucose concentration in the blood serum decreased, favorable changes in body weight occurred, transaminase activity and lipid profile improved in streptozotocin-induced diabetic rats using gold nanoparticles synthesized by Cassia fistula stem bark extract [94][253]. In vitro results showed that AuNPs not only improved insulin secretion induced by di-(2-ethylhexyl) phthalate (DEHP) (DEHP played as a diabetogenic agent by increasing free radicals and decreasing insulin levels finally resulting in loss of pancreatic cells mass) but also protected RIN-5F cells (a clone derived from the RIN-m rat islet line) from toxicity caused by DEHP by increasing cell viability and insulin secretion. AuNPs also prevent oxidative cells damage and normalize the regulation of Bcl-2 (Bcl-2 is a regulatory protein, is involved in apoptotic regulation) family proteins through an unregulated insulin signaling pathway [95][96][254,255]. In addition, the antidiabetic activity of AuNPs from Fritillaria cirrohosa was shown in preclinical models [97][256]. Gold nanoparticles from Ziziphus jujuba can diminish diabetes complications by lipid peroxidation and oxidative stress decline [98][257]. Using gold nanoparticles can become the basis for diabetic nephropathy treatment [99][258]. All these data characterize AuNPs as excellent hypoglycemic agents in diabetes mellitus therapy and related complications.
Leishmanicidal activity. The main vectors of Dengue fever and malaria–Aedes aegypti and Anopheles stephensi mosquitoes represent a very significant threat to the tropical and subtropical population. Gold nanoparticles can help in solving this problem as well. The larvicidal activity was shown for AuNPs from Jasminum nervosum leaf extract against filarial and arbovirus vector Culex quinquefasciatus [100][259], against larvae and pupae of the malaria vector A. stephensi and the dengue vector A. aegypti [101][260].
Photothermal therapy. Photothermal therapy is a minimally invasive technique, which uses hyperthermia generated by photothermal agents from laser energy to kill cancer cells [102][261]. Hyperthermia was known as one of the most effective radiosensitizers [103][262]. The nanotechnological idea is to deliver AuNPs specifically to a tumor, apply NIR (near-infrared spectroscopy) light that will predominantly heat only the tumor, and then deliver radiotherapy [104][263]. Potential gold nanoparticle hyperthermia approaches in cancer treatment may have various advantages [104][263]: (a) they can be activated via near-infrared (NIR) laser light, creating the ability to penetrate deep into biological tissues; (b) a radiotherapy and hyperthermia combination can lead to higher effectiveness than the use of radiotherapy alone; (c) they can reduce the radiotherapy dose and make it more tumor-specific; (d) direct infusion can reduce common toxicity effect; (e) they can be modified to create multidimensional cancer photothermal therapy and drug delivery systems [105][106][264,265]. AuNPs-mediated photothermal therapy combined with checkpoint immunotherapy was discovered to reverse tumor-mediated immunosuppression, thereby leading to the treatment of primary tumors [107][266]. Green-synthesized curcumin-coated gold nanoparticles can induce apoptotic cell death in photothermal therapy and radiofrequency electric field hyperthermia [108][267]. Unfortunately, the data about biosynthesized gold nanoparticles and their application in this matter are practically absent.
Drug delivery. Gold nanoparticles can be used as a delivery method for various therapeutic agents. Molecules with different functional groups can bind with high affinity on the surface of AuNPs. Capping agents surrounding the AuNPs can be displaced by other functioning thiols or adsorbed ligands through a ligand exchange reaction [109][38]. AuNPs can bind with other materials covalently and non-covalently [109][38]. Covalent conjugation stabilizes the conjugates for imaging. Electrostatic interactions, hydrophobic interactions, and specific binding affinity can act as non-covalently binding with AuNPs [109][38]. Gold nanoparticles can be functionalized by different compounds carrying the healing effect. Coating molecules (for instance, PEG and BSA) are attached to provide a binding surface for specific cells, minimizing, in that way, non-specific targeting on other tissues [110][268]. For example, PEGylation of gold nanoparticles can minimize macrophages and monocytes uptake, providing them with a cover and prolonging their availability and concentration in tumor tissue [111][269]. Not only small molecular drugs but also large biomolecules (such as DNA, RNA, peptides, and proteins) are delivered by AuNPs [110][268]. Anticancer drugs such as doxorubicin, 5-Fluorouracil may be target compounds in delivery by AuNPs [112][78][113][114][86,237,270,271]. Biosynthesized AuNPs are also used as drug delivery system for cancer therapy in a mouse model [115][272]. AuNPs modified with tryptophan and 5-aminopurine have excellent antibacterial activity against multidrug-resistant bacteria [116][273]. Green gold nanoparticles are particularly interesting because, having their capping agents with useful properties, they can be equipped with additional molecules to achieve and increase the therapeutic effect.
Bio-sensing and Detection. According to their properties, AuNPs can be used in biosensing. Perfect sensitivity in determining cancerous cells, biological molecules, blood glucose levels, bacteria, viruses, toxins, and pollutants is proved by gold nanoparticles [117][274]. The optical and electronic properties of AuNPs are used in various cell imaging techniques, such as computed tomography, dark-field microscopy, optical coherence tomography, and Raman spectroscopy. AuNPs properties such as colorimetric, surface plasmon resonance, electrical, electrochemical, and fluorescence can be the base for different kinds of sensors [118][275]. AuNPs play a crucial role in the analysis called “bio-barcode assay” [119][276]. This analysis is an ultrasensitive method for detecting target proteins and nucleic acids. The bio-barcode assays are generally based on AuNPs functionalization with many strands of oligonucleotides strands (“barcodes”) and a corresponding recognition agent which can be antibody in terms of protein detection, and a small segment of the barcoded strand in case of nucleic acids detection [119][276]. Gold nanoparticles are often used as amplifiers in SPR sensors. An important advantage of metal nanoparticles is the dual mechanism of SPR enhancing [120][121][122][277,278,279]. Enhancing of the PPR sensor signal was proposed by Kao et al. in the determination of antibodies against glutamic acid decarboxylase—GAD (glutamic acid decarboxylase—GAD), a marker for the diagnosis of insulin-dependent diabetes [123][280]. This approach allows decreasing the detection limit of antibodies by four orders of magnitude [123][280]. The enhanced fluorescent properties of AuNPs have made the detection of aflatoxins easier [124][281]. AuNPs are of great interest in the colorimetric detection of viruses [125][282]. The approach is based on the two main techniques: (1) a color amplification technique in which AuNPs are applied to act as direct coloring labels with their characteristic, intense red color; (2) a color changes technique in which a color change from red to purple occurs in response to particle aggregation [126][127][283,284]. Gold nanoparticles are applied in microorganisms detection [110][268]. AuNPs functionalized by oligonucleotides complementary to the unique sequences of the heat-shock protein 70 (HSP 70) of Cryptosporidium parvum was used to detect the oocytes of Cryptosporidium in a colorimetric assay [128][285]. Staphylococcus enterotoxin B was detected by gold nanoparticle-based chemiluminescence assay [129][286].
