3. Reproductive Axis at the Interface with Environmental Obesogens
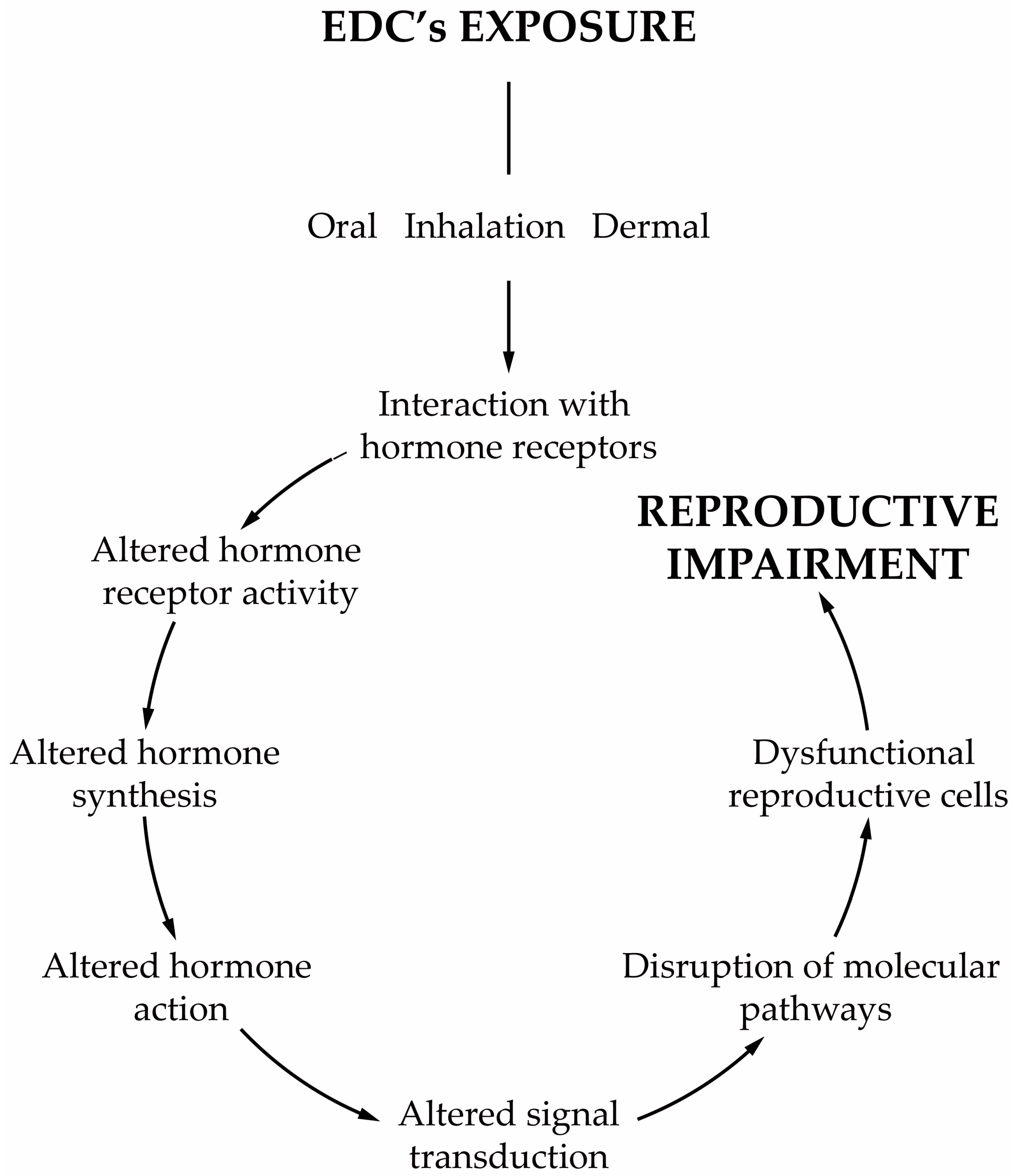
The central neuroendocrine system controls all processes in the body, reproduction included. All molecular events are initiated at the base of the brain, where the hypothalamus is located, which serves as the primary interface between the central nervous system and the testicles, in this case. The hypothalamic neural cells synthesize and release the gonadotropin-releasing hormone (GnRH) into the capillary portal system that vascularizes the anterior pituitary. The gonadotrophs in the pituitary response to this stimulus is the release of the corresponding hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the bloodstream. FSH and LH act as a functional link between the brain and testes by working on testicular cells to regulate the spermatogenic event
[30][134]. LH binds to membrane receptors of LCs and stimulates T synthesis, influencing the development of peritubular cells, SCs and, consequently, germ cells
[31][135]. LCs irreversibly convert T into
17β-estradiol (E
2) by aromatase P450
[32][136]. On the other hand, FSH binds to membrane receptors on SCs, stimulating the production of E
2, activin and inhibin B. E
2 inhibits T production by LCs, while activin and inhibin B produce a positive/negative feedback on the pituitary, respectively. There is evidence that endocrine disruptors affect the neuroendocrine systems, and most of the studies have focused on the hypothalamus–pituitary–testicular (HPT) axis, termed the reproductive axis. Endocrine disruptors can exert diverse actions over the target cells according to their chemical structure and activities. In vitro evidences demonstrate that GnRH GT1–7 cell lines exposed to low doses of PCB mixtures, Aroclor 1221 or Aroclor 1254, increased the expression of the GnRH gene
[33][137]. In this case, the endocrine disruption occurs at the level of the hypothalamus
[33][137]. Similar studies using GT1–7 cell lines exposed to small doses of organochlorine pesticides demonstrated an increased expression of GnRH, whereas when exposed to high doses, the opposite effect was observed
[34][138]. Although these works showed a positive response of GnRH cell lines to environmental obesogens, other studies demonstrated different outcomes. In fact, explanted hypothalamus from male rats exposed in utero to dioxins displayed an increased content of GnRH peptide, which was, however, accompanied by an impaired release
[35][139]. Obesogens can favor or reduce the release of neuroendocrine hormones, but this will depend on the type of obesogens, the time of exposure and perhaps the levels to which disruption in the reproductive axis occurs. Different environmental compounds may target the HPT axis at various sites with different intensities, disrupting its regulation.
4. Leydig Cells Are a Sensitive Target of Obesogens
The synthesis of sex steroid hormones occurs in the Leydig cells (LCs) which are extremely sensitive to the toxic effects of environmental contaminants. The deleterious effects of EDCs often conduct the impairment of hormone synthesis since they are associated with the inhibition of the activities of enzymes involved in steroidogenesis. Lipids are essential for this process and cholesterol is one of the primary precursors, so it would be expected that any dysregulation in the homeostasis of these components compromises the synthesis of steroid hormones. Indeed, the pesticide 2,4-dichlorophenoxyacetic acid (2,4-D) decreases the expression of 3-hydroxy-3-methylglutaryl coenzyme A synthase (HMG-CoA synthase) and reductase (HMG-CoA reductase) in LCs of mice
[36][152]. These enzymes are pivotal for cholesterol synthesis, where HMG-CoA synthase is responsible for the condensation of acetyl-CoA with acetoacetyl-CoA to form HMG-CoA, which is reduced to mevalonate for the synthesis of cholesterol. This is one of the most important steps of the cholesterol biosynthesis, where the enzyme responsible for this committing conversion is HMG-CoA reductase. The impairment of cholesterol synthesis after exposure to 2,4-D disturbs testosterone synthesis. In the steroidogenesis, cholesterol is then carried from the outer mitochondrial membrane by steroid acute regulatory protein (StAR), where it is converted to pregnenolone via P450 side chain cleavage enzyme (P450scc). Then, this intermediate proceeds to the smooth endoplasmic reticulum where 3β-hydroxysteroid dehydrogenase (3β-HSD) turns it into progesterone. Progesterone is converted by P450c17 into 17-hydroxyprogesterone and into androstenedione, where the enzyme 17β-hydroxysteroid dehydrogenase converts it into T. It has been demonstrated that exposure to plasticizers such as di(n-butyl)phthalate decreased the expression of cholesterol transport genes such as StAR, the high-density lipoprotein receptor, also known as SRB1
[37][153]. Furthermore, the expression of genes involved in T biosynthesis, namely P450scc, 3β-HSD, and P450c17, was downregulated, which probably contributes to T deficiency
[38][154]. It seems that the plasticizer DEHP exerts anti-androgenic effects directly onto LCs, inhibiting T synthesis probably through dysfunction of CYP17
[39][155]. However, the impact of its metabolite, MEHP, is much more pronounced since it inhibited the expression of all steroidogenic enzymes, as well as all T precursors, in both the Δ4 and Δ5 steroidogenic pathways
[39][155]. The effects of both phthalates appeared to be specific for steroidogenesis since they did not alter the expression of the insulin-like 3 gene, a specific marker of LCs. In vivo models have also provided evidence of the cytotoxic effects of similar obesogens in testicular function as observed after daily exposure to 200 mg/kg/day of BPA for six weeks. Such exposure not only revealed the same results, but also disclosed a reduced number of LCs
[40][156].
5. Mechanisms Mediating Obesogen-Related Sertoli Cell Dysfunction: A Metabolic Standpoint
Typically known as “nurse cells”, Sertoli cells (SCs) are extremely important in male fertility, ensuring all physical and nutritional support of the germ cell line. Spermatogenesis is under the strict control of hormonal and endogenous factors
[41][42][165,166], so SCs become an easy target of obesogenic EDCs mimicking endogenous hormones
[43][167]. EDCs (obesogens included), not only compromise all structure of SCs, but also induce various cellular and molecular insults
[44][45][168,169]. Additionally, the interactions between adjacent SCs are disrupted, provoking the premature exfoliation of germ cells
[46][170]. The impact of EDCs on the integrity of cytoskeleton disruption of SCs and BTB has been extensively explored and well documented by Cheng and his collaborators
[18][29][45][47][48][49][50][51][52][22,120,169,171,172,173,174,175,176].
6. How Can Germ Cells Be Affected by Obesogens?
Compelling evidence shows that environmental contaminants induce several dysfunctions associated with the testicular dysgenesis syndrome, including seminiferous tubules atrophy and germ cell degeneration
[53][185]. Furthermore, lipids are essential components of the membranes of germ cells. Deficient lipid incorporation into these cells leads to a defective germ cell structure and contributes to the impairment of sperm parameters, and consequently to alterations of sperm functionality. The oral administration of TBT has also been associated with induced apoptosis in testicular germ cells in prepubertal mice
[28][119]. Those authors did not describe the molecular mechanisms by which germ cell apoptosis was induced, but according to recent evidence, it seems that environmental contaminants activate both intrinsic and extrinsic pathways of apoptosis. Mitra and collaborators
[54][133] observed a caspase-3 activation and increased levels of pathways of phosphorylated c-Jun N-terminal kinase (JNK) and mitogen-activated protein kinase p-38 (p38-MAPK) in SC–germ cell co-cultures after being exposed to 600 nM of TBT. JNK activation serves as a pro-apoptotic signal and regulates the mitochondrial apoptotic pathway through a balancing act between the activation of pro-apoptotic members and inhibition of anti-apoptotic members of the Bcl2-related protein family
[55][186]. On the other hand, the p-38 caspase-independent pathway seems to be also activated by TBT and thus plays a role in the germ cells’ death
[56][187].